-
Name
GLYCOLALDEHYDE
- EINECS
- CAS No. 141-46-8
- Article Data333
- CAS DataBase
- Density 1.065g/cm3
- Solubility
- Melting Point 97°C
- Formula C2H4 O2
- Boiling Point 131.3°Cat760mmHg
- Molecular Weight 60.0526
- Flash Point 42°C
- Transport Information
- Appearance
- Safety A poison by intraperitoneal route. Moderately toxic by ingestion. Mutation data reported. When heated to decomposition it emits acrid smoke and irritating vapors.
- Risk Codes
-
Molecular Structure
- Hazard Symbols Moderately toxic by ingestion.
- Synonyms Glycolaldehyde(8CI); 2-Hydroxyacetaldehyde; 2-Hydroxyethanal; 2-Oxoethanol; Diose; Glycolicaldehyde; Hydroxyacetaldehyde; Hydroxyethanal; Methylolformaldehyde;Monomethylolformaldehyde; NSC 67935
- PSA 37.30000
- LogP -0.82240
Synthetic route

| Conditions | Yield |
|---|---|
| With N,N-dibutylacetamide; trimethylbenzoic acid; acetylacetonatodicarbonylrhodium(l); 2-phospha-2-(2-N,N-dimethylcarbamoylethyl)-1,3,5,7-tetramethyl-6,9,10-trioxatricyclo[3.3.1.1(3,7)]decane In water at 90℃; for 5h; Product distribution / selectivity; | 90% |
| With sodium hydroxide; Wang resin-pentylstyrene[5-Ph(4Me)]N-Me-imidazole(1+)*Cl(1-) In tetrahydrofuran for 0.166667h; Heating; | 77% |
| With 1-methyl-pyrrolidin-2-one; trimethylbenzoic acid; acetylacetonatodicarbonylrhodium(l); 6-phospha-2,4,8-trioxa-1,3,5,7-tetramethyladamantyl(n-C20H41) at 110℃; for 2h; Product distribution / selectivity; | 76% |
-
-
50-00-0
formaldehyd

-
-
16842-03-8, 64519-62-6
hydridocobalt tetracarbonyl

-
A
-
67-56-1
methanol

-
B
-
15226-74-1, 61091-28-9, 61117-58-6
dicobalt octacarbonyl

-
C
-
107-31-3
Methyl formate

-
D
-
107-21-1
ethylene glycol

-
E
-
141-46-8
Glycolaldehyde

| Conditions | Yield |
|---|---|
| In dichloromethane reaction of gaseous formaldehyde with excess of HCo(CO)4 in CH2Cl2 (0°C, 1 h), color of soln. turning brown; IR, 1H NMR; | A 80% B n/a C 0% D 0% E 0% |


| Conditions | Yield |
|---|---|
| at 358℃; for 0.000833333h; Product distribution; Curie-point pyrolysis; | A 15.3% B 74% C 2% |

-
-
131543-46-9
Glyoxal

-
-
141-46-8
Glycolaldehyde

| Conditions | Yield |
|---|---|
| 73.6% | |
| 70% |
-
-
532-20-7, 613-83-2, 7261-25-8, 7687-39-0, 13221-22-2, 14795-83-6, 15761-67-8, 20074-49-1, 25545-03-3, 25545-04-4, 32445-75-3, 34436-17-4, 36441-93-7, 36468-53-8, 37110-85-3, 37388-49-1, 38029-69-5, 40461-77-6, 40461-89-0, 41546-19-4, 41546-20-7, 41546-21-8, 41546-26-3, 41546-29-6, 41546-30-9, 41546-31-0, 126872-16-0, 131064-98-7
D-Ribose

-
A
-
98-01-1
furfural

-
B
-
141-46-8
Glycolaldehyde

-
C
-
116-09-6
hydroxy-2-propanone

| Conditions | Yield |
|---|---|
| at 358℃; for 0.000833333h; Product distribution; Curie-point pyrolysis; | A 71.7% B 12.4% C 5.4% |


| Conditions | Yield |
|---|---|
| With periodate; permanganate(VII) ion In water | A 10% B 19% C 71% |


| Conditions | Yield |
|---|---|
| at 358℃; for 0.000833333h; Product distribution; Curie-point pyrolysis; | A 3.2% B 69.8% C 3.1% D 8.6% |


| Conditions | Yield |
|---|---|
| In water at 500℃; for 5.64h; Catalytic behavior; Reagent/catalyst; | 69.3% |
| With water; magnesium carbonate | |
| With water; calcium carbonate |
-
-
28697-53-2
D-arabinopyranose

-
A
-
98-01-1
furfural

-
B
-
141-46-8
Glycolaldehyde

-
C
-
116-09-6
hydroxy-2-propanone

| Conditions | Yield |
|---|---|
| at 358℃; for 0.000833333h; Product distribution; Curie-point pyrolysis; | A 66.6% B 24.3% C 3.9% |

-
-
6347-01-9
fructopyranose

-
A
-
98-01-1
furfural

-
B
-
141-46-8
Glycolaldehyde

-
C
-
116-09-6
hydroxy-2-propanone

| Conditions | Yield |
|---|---|
| at 358℃; for 0.000833333h; Product distribution; Curie-point pyrolysis; | A 65% B 17.4% C 6.4% |

-
-
50-00-0
formaldehyd

-
-
201230-82-2
carbon monoxide

-
A
-
67-56-1
methanol

-
B
-
64-18-6
formic acid

-
C
-
141-46-8
Glycolaldehyde

| Conditions | Yield |
|---|---|
| With rhodium(III) chloride; water; hydrogen; triphenylphosphine In N,N-dimethyl acetamide at 109.9℃; under 90007.2 Torr; for 1h; Thermodynamic data; Equilibrium constant; Product distribution; ΔG; further rhodium complexes, solvents, pressure of reagents; | A n/a B n/a C 65% |


| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 120℃; under 15751.6 Torr; for 1h; Temperature; Pressure; Reagent/catalyst; Solvent; | A 63.52% B 23.12% |
| With triethylamine In N,N-dimethyl-formamide at 100℃; under 3750.38 Torr; for 1h; Temperature; Pressure; Reagent/catalyst; Solvent; | A 25.17% B 38.23% |
| With 5-methoxy-1,3,4-triphenyl-4,5-dihydro-1H-1,2-4-triazoline In various solvent(s) at 80℃; |
-
-
530-26-7
D-Mannose

-
A
-
110-00-9
furan

-
B
-
98-01-1
furfural

-
C
-
141-46-8
Glycolaldehyde

-
D
-
116-09-6
hydroxy-2-propanone

| Conditions | Yield |
|---|---|
| at 358℃; for 0.000833333h; Product distribution; Curie-point pyrolysis; | A 2.2% B 63.4% C 18.4% D 7.2% |


| Conditions | Yield |
|---|---|
| at 358℃; for 0.000833333h; Product distribution; Curie-point pyrolysis; | A 4.2% B 62.7% C 6.4% D 6.3% |

-
-
132531-72-7
1-(phenylsulfenyl)-1,2-bis(trifluoroacetoxy)ethane

-
-
141-46-8
Glycolaldehyde

| Conditions | Yield |
|---|---|
| With triethylamine In methanol; dichloromethane at 0℃; for 0.0833333h; | 62% |
-
-
87-72-9, 608-45-7, 608-46-8, 608-47-9, 2460-44-8, 6748-95-4, 6763-34-4, 7261-26-9, 7283-06-9, 7283-07-0, 7296-55-1, 7296-56-2, 7296-58-4, 7296-59-5, 7296-60-8, 7296-61-9, 7296-62-0, 7322-30-7, 10257-31-5, 10257-32-6, 10257-33-7, 10257-34-8, 10257-35-9, 19982-83-3, 20242-88-0, 28697-53-2, 36562-42-2, 41546-41-2, 89299-64-9, 107655-34-5, 115794-06-4, 115794-07-5, 130550-15-1, 130606-21-2
D-Xylose

-
A
-
98-01-1
furfural

-
B
-
141-46-8
Glycolaldehyde

-
C
-
116-09-6
hydroxy-2-propanone

| Conditions | Yield |
|---|---|
| at 358℃; for 0.000833333h; Product distribution; Curie-point pyrolysis; | A 61.7% B 28.6% C 4% |

-
-
73-34-7
L-rhamnose

-
A
-
98-01-1
furfural

-
B
-
75-07-0
acetaldehyde

-
C
-
141-46-8
Glycolaldehyde

-
D
-
116-09-6
hydroxy-2-propanone

| Conditions | Yield |
|---|---|
| at 358℃; for 0.000833333h; Product distribution; Curie-point pyrolysis; | A 2.2% B 19.4% C 15.2% D 59.9% |

-
-
821-11-4, 6117-80-2, 110-64-5
1,4-butenediol

-
-
141-46-8
Glycolaldehyde

| Conditions | Yield |
|---|---|
| With ozone In methanol at -78℃; | 58% |

| Conditions | Yield |
|---|---|
| at 235 - 590℃; for 0.000833333h; Product distribution; Curie-point pyrolysis, yield at 358 deg C; | A 56.4% B 24.4% C 8.8% |

-
-
87-72-9, 608-45-7, 608-46-8, 608-47-9, 2460-44-8, 6748-95-4, 6763-34-4, 7261-26-9, 7283-06-9, 7283-07-0, 7296-55-1, 7296-56-2, 7296-58-4, 7296-59-5, 7296-60-8, 7296-61-9, 7296-62-0, 7322-30-7, 10257-31-5, 10257-32-6, 10257-33-7, 10257-34-8, 10257-35-9, 19982-83-3, 20242-88-0, 28697-53-2, 36562-42-2, 41546-41-2, 89299-64-9, 107655-34-5, 115794-06-4, 115794-07-5, 130550-15-1, 130606-21-2
D-Xylose

-
A
-
98-01-1
furfural

-
B
-
96-26-4
dihydroxyacetone

-
C
-
141-46-8
Glycolaldehyde

-
D
-
78-98-8
2-oxopropanal

| Conditions | Yield |
|---|---|
| With SO4(2-)/12 wt percent ZrO2-Al2O3/SBA-15 In water; toluene at 160℃; for 4h; | A 52.7% B n/a C n/a D n/a |

-
-
72-87-7, 1136-42-1, 1136-44-3, 6730-06-9, 7772-94-3, 10036-64-3, 14131-60-3, 14131-64-7, 14131-68-1, 14215-68-0, 62057-49-2, 62057-50-5, 62057-51-6, 62057-52-7, 62057-53-8, 62057-54-9, 62057-55-0, 62057-56-1, 62075-50-7, 62137-54-6, 134451-97-1, 134522-12-6, 148347-16-4, 6082-29-7
N-acetyl-D-galactosamine

-
A
-
98-01-1
furfural

-
B
-
67-56-1
methanol

-
C
-
64-17-5
ethanol

-
D
-
141-46-8
Glycolaldehyde

| Conditions | Yield |
|---|---|
| at 358℃; for 0.000833333h; Product distribution; Curie-point pyrolysis; | A 50.2% B 2.9% C 8.6% D 24.1% |


| Conditions | Yield |
|---|---|
| With hydrogen; RhCl(PPh3)3 In N,N-dimethyl-formamide at 110℃; under 97507.8 Torr; for 2h; | 50% |
| With hydrogen; phenol; dicobalt octacarbonyl; Ru(CO)12 In water at 60℃; for 6h; | |
| With hydrogen In N,N-dimethyl acetamide at 99.9℃; under 97507.8 Torr; for 1h; Mechanism; in the presence of RhCl(PPh3) and other Rh complexes; IR monitoring; |

| Conditions | Yield |
|---|---|
| With potassium pyrophosphate In water at 4℃; for 16h; | A n/a B 50% |

| Conditions | Yield |
|---|---|
| at 358℃; for 0.000833333h; Product distribution; Curie-point pyrolysis; | A 39.8% B 48.5% C 5.1% |


| Conditions | Yield |
|---|---|
| With sodium hydroxide; N-bromoacetamide at 35℃; Rate constant; | 48.4% |
| With dihydrogen peroxide; iron(II) sulfate | |
| With sodium hypochlorite; water |
-
-
10257-28-0
D-Galactose

-
A
-
98-01-1
furfural

-
B
-
67-56-1
methanol

-
C
-
141-46-8
Glycolaldehyde

-
D
-
116-09-6
hydroxy-2-propanone

| Conditions | Yield |
|---|---|
| at 235 - 590℃; for 0.000833333h; Product distribution; Curie-point pyrolysis, yield at 358 deg C; | A 47.2% B 5.6% C 31.9% D 4.9% |

-
-
7512-17-6, 72-87-7, 1136-42-1, 1136-44-3, 6082-29-7, 6730-06-9, 7772-94-3, 10036-64-3, 14131-60-3, 14131-64-7, 14131-68-1, 14215-68-0, 62057-49-2, 62057-50-5, 62057-51-6, 62057-52-7, 62057-53-8, 62057-54-9, 62057-55-0, 62057-56-1, 62075-50-7, 62137-54-6, 134451-97-1, 134522-12-6, 148347-16-4
N-Acetyl-D-glucosamine

-
A
-
98-01-1
furfural

-
B
-
141-46-8
Glycolaldehyde

| Conditions | Yield |
|---|---|
| at 358℃; for 0.000833333h; Product distribution; Curie-point pyrolysis; | A 44.5% B 46.4% |

| Conditions | Yield |
|---|---|
| With sodium chlorite; dimethyl sulfoxide In aq. phosphate buffer at 0 - 20℃; pH=4; | 100% |
| With 5 % platinum on carbon In water at 80℃; under 7500.75 Torr; for 6h; Temperature; Time; Reagent/catalyst; Autoclave; | 78% |
| With oxygen In water at 180℃; under 3750.38 Torr; for 1h; Autoclave; | 50% |

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In chloroform at -20℃; for 14h; | 100% |

| Conditions | Yield |
|---|---|
| 1-hydroxytetraphenylcyclopentadienyl(tetraphenyl-2,4-cyclopentadien-1-one)-μ-hydrotetracarbonyldiruthenium(II) In 1,4-dioxane; toluene at 20℃; for 5h; Product distribution / selectivity; Heating / reflux; | 100% |

| Conditions | Yield |
|---|---|
| With C27H36BN3 In methanol at 25℃; for 24h; nitro Aldol reaction; Inert atmosphere; | 99% |
| With sodium methylate In methanol; dimethyl sulfoxide for 20h; | 60% |
-
-
2319-57-5
threitol

-
-
141-46-8
Glycolaldehyde

-
-
627895-26-5
(2R,6R,9S,10S)-2,6-bis(hydroxymethyl)-cis-1,3,5,7-tetraoxadecalin

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 0.25h; | 99% |
-
-
14321-27-8
N-ethylbenzylamine

-
-
141-46-8
Glycolaldehyde

-
-
83947-56-2
4,4,5,5-tetramethyl-2-((E)-styryl)-[1,3,2]dioxaborolane

| Conditions | Yield |
|---|---|
| In various solvent(s) at 20℃; for 4h; Petasis reaction; | 99% |

-
-
2418-52-2
D-threitol

-
-
141-46-8
Glycolaldehyde

-
-
173950-19-1
(2S,9R,6S,10R)-2,6-Di(hydroxymethyl)-1,3,5,7-tetraoxadecalin

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 0.25h; | 98% |

| Conditions | Yield |
|---|---|
| With triethylamine In diethyl ether at 60℃; for 17h; Sealed tube; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With tributylphosphine; carbon monoxide; hydrogen; acetylacetonatodicarbonylrhodium(l) In various solvent(s) at 80℃; under 73505.8 Torr; for 1h; Product distribution; other solvent (DMI); other reactn. time; other catalysts; | 97% |
| With hydrogen; nickel In water at 40℃; under 37503.8 Torr; for 15h; | 90% |
| durch Einw.gaerender Hefe; |

| Conditions | Yield |
|---|---|
| With oxygen In water glycerine phosphate oxidase 70 U GPO, catalase 1000 U Cat, L-rhamnulose-1-phosphate aldolase 50 U RhuA; | 96% |
-
-
110-91-8
morpholine

-
-
141-46-8
Glycolaldehyde

-
-
83947-56-2
4,4,5,5-tetramethyl-2-((E)-styryl)-[1,3,2]dioxaborolane

| Conditions | Yield |
|---|---|
| In various solvent(s) at 20℃; for 4h; Petasis reaction; | 96% |


| Conditions | Yield |
|---|---|
| In aq. buffer for 24h; pH=7; Reagent/catalyst; | 95% |
| In water at 20℃; pH=7; |
-
-
13331-23-2
fur-2-ylboronic acid

-
-
3290-99-1
4-methoxybenzoic acid hydrazide

-
-
141-46-8
Glycolaldehyde

-
-
1354376-72-9
C14H16N2O4

| Conditions | Yield |
|---|---|
| In methanol Petasis Reaction; | 95% |


| Conditions | Yield |
|---|---|
| With oxygen In water glycerine phosphate oxidase 70 U GPO, catalase 1000 U Cat, L-fuculose-1-phosphate aldolase FucA; | 93% |

| Conditions | Yield |
|---|---|
| With manganese; chromium; hydrogen; nickel; aluminium In water at 150℃; under 22502.3 Torr; Temperature; Pressure; Reagent/catalyst; Solvent; Autoclave; | 92.3% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 4h; | 92.1% |

| Conditions | Yield |
|---|---|
| 92% |
-
-
141-46-8
Glycolaldehyde

-
-
21204-67-1
methyl (triphenylphosphoranylidene)acetate

-
-
29576-13-4
methyl 4-hydroxy-2-butenoate

| Conditions | Yield |
|---|---|
| In benzene for 4h; Heating; | 91% |
| In benzene at 100℃; for 5h; | 44% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 20℃; for 0.25h; | 91% |
-
-
134258-38-1
tert-butyl (2S,4S,5S)-5-acetamido-4-<(tert-butyl)dimethylsilyloxy>piperidine-2-carboxylate

-
-
141-46-8
Glycolaldehyde

-
-
134258-39-2
tert-butyl (2S,4S,5S)-5-acetamido-4-<(tert-butyl)dimethylsilyloxy>-1-(2-hydroxyethyl)piperidine-2-carboxylate

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal In methanol under 760 Torr; for 12h; Ambient temperature; | 90% |

| Conditions | Yield |
|---|---|
| With fructose 1,6-bisphosphate aldolase from S. carnosus; triose phosphate isomerase from rabbit muscle [EC 5.3.1.1] In water at 20℃; Addition; Aldol condensation; Enzymatic reaction; | 90% |
-
-
929281-33-4
3-{[(1R)-1-(2-chlorophenyl)ethyl]oxy}-5-[6-(4-piperidinyl)-1H-benzimidazol-1-yl]-2-thiophenecarboxamide

-
-
141-46-8
Glycolaldehyde

| Conditions | Yield |
|---|---|
| With sodium tris(acetoxy)borohydride; acetic acid In methanol; dichloromethane; water at 20℃; for 3h; | 90% |

| Conditions | Yield |
|---|---|
| With transaldolase B F178Y/R181E In water-d2 at 25℃; for 24h; Kinetics; Reagent/catalyst; Aldol reaction; Enzymatic reaction; stereoselective reaction; | A 8% B 90% |


| Conditions | Yield |
|---|---|
| With Escherichia coli D-fructose-6-phosphate aldolase L107Y/A129G mutant for 5h; Enzymatic reaction; stereoselective reaction; | 90% |
-
-
141-46-8
Glycolaldehyde

| Conditions | Yield |
|---|---|
| With diamidophosphate In aq. phosphate buffer at 20℃; for 4h; pH=4; pH-value; | 90% |

| Conditions | Yield |
|---|---|
| With zirconium(IV) chloride In water-d2 at 20℃; for 5h; | 90% |

| Conditions | Yield |
|---|---|
| With Pseudomonas putida trans-o-hydroxybenzylidenepyruvate hydratase-aldolase Enzymatic reaction; stereoselective reaction; | 90% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; magnesium(II) chloride hexahydrate; transketolase H102L/H474S mutant from Geobacillus stearothermophilus; thiamine pyrophosphate; sodium hydroxide In water at 50℃; for 12h; pH=7.5; Kinetics; Reagent/catalyst; Enzymatic reaction; enantioselective reaction; | 88% |
| With Escherichia coli 1-deoxy-D-xylulose-5-phosphate synthase; thiamine diphosphate; magnesium chloride In aq. phosphate buffer pH=7.5; Kinetics; Enzymatic reaction; |
-
-
1622204-19-6
6-(2-methoxyethoxy)-N-methyl-5-{[2-({[4-(piperidin-4-yl)phenyl]carbonyl}amino)pyridin-4-yl]oxy}-1H-indole-1-carboxamide

-
-
141-46-8
Glycolaldehyde

-
-
1622204-21-0
(5-({2-[({4-[1-(2-hydroxyethyl)piperidin-4-yl]phenyl}carbonyl)amino]pyridin-4-yl}oxy)-6-(2-methoxyethoxy)-N-methyl-1H-indole-1-carboxamide)

| Conditions | Yield |
|---|---|
| With sodium tris(acetoxy)borohydride In tetrahydrofuran at 20℃; for 3.75h; | 87% |
| With sodium tris(acetoxy)borohydride In tetrahydrofuran at 20℃; for 2h; | 83% |
GLYCOLIC ALDEHYDE Chemical Properties
The molecular structure of glycolic aldehyde(141-46-8):
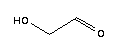
This glycolic aldehyde(141-46-8)'s structure is also available as a 2d Mol file or as a computed 3d Mol file.
GLYCOLIC ALDEHYDE Toxicity Data With Reference
| 1. | mic-sat 12500 nmol/plate | JCROD7 Journal of Cancer Research and Clinical Oncology. 111 (1986),149. | ||
| 2. | mrc-orl-uns-dmg 125 mmol/L | CRNGDP Carcinogenesis. 17 (1996),1083. | ||
| 3. | dnd-hmn-lym 5 mmol/L | MUREAV Mutation Research. 304 (1994),229. | ||
| 4. | orl-rat LDLo:3 g/kg | AJCPAI American Journal of Clinical Pathology. 45 (1966),46. | ||
| 5. | ipr-rat LD50:280 mg/kg | TXAPA9 Toxicology and Applied Pharmacology. 71 (1983),84. |
GLYCOLIC ALDEHYDE Safety Profile
Related Products
- Glycolic acid
- Glycolic acid, calcium salt
- GLYCOLIC ALDEHYDE
- 141474-37-5
- 141483-15-0
- 14148-42-6
- 141489-66-9
- 141492-50-4
- 141492-94-6
- 14149-39-4
- 141505-33-1
- 14150-95-9
- 141516-24-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View