-
Name
PERFLUOROPROPYL IODIDE
- EINECS 212-045-5
- CAS No. 754-34-7
- Article Data26
- CAS DataBase
- Density 2.189 g/cm3
- Solubility
- Melting Point -95°C
- Formula C3F7I
- Boiling Point 37.9 °C at 760 mmHg
- Molecular Weight 295.926
- Flash Point 3.4 °C
- Transport Information
- Appearance light red liquid
- Safety 23-24/25
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi;
Xi; T
T
- Synonyms Propane,heptafluoro-1-iodo- (6CI,7CI,8CI);1,1,1,2,2,3,3-Heptafluoro-3-iodopropane;1,1,2,2,3,3,3-Heptafluoro-1-iodopropane;1-Iodoheptafluoropropane;1-Iodoperfluoropropane;Heptafluoro-1-iodopropane;Heptafluoropropyl iodide;NSC 66409;Perfluoropropyl iodide;R 217I1;n-Heptafluoropropyl iodide;
- PSA 0.00000
- LogP 3.21180
Synthetic route
-
-
1349736-92-0
[(η5-pentamethylcyclopentadienyl)2Rh2I(μ-I)2(CH2CH2CF(CF3)2)]

-
-
1350458-19-3
[(η5-pentamethylcyclopentadienyl)2Rh2(μ-I)2(CH2CH2CF(CF3)2)2]*benzene-d6

-
-
603-35-0
triphenylphosphine

-
A
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
B
-
1349737-19-4
[(η5-pentamethylcyclopentadienyl)RhI(CH2CH2CF(CF3)2)(PPh3)]

| Conditions | Yield |
|---|---|
| In tetrahydrofuran byproducts: CH2CH2; (N2, Schlenk technique); stirring mixt. of rhodium compds. and phosphinederiv. in THF for 5 h; evapn. in vac., chromy. (silica gel, diethyl ether/hexane (1:1)), evapn., elem. anal.; | A n/a B 86% |

-
-
678-07-9
heptafluoropropyl-diiodo-phosphine

-
A
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
B
-
756-18-3
bis-heptafluoropropyl-iodo-phosphine

-
C
-
13455-01-1
phosphorous triiodide

| Conditions | Yield |
|---|---|
| 220°C (48 h); | A 46% B 36% C n/a |

-
-
105062-53-1
C3F17IO2Te2

-
A
-
76-19-7
freon-218

-
B
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
C
-
91600-26-9
C3F12OTe

| Conditions | Yield |
|---|---|
| at 115℃; for 26h; | A n/a B n/a C 30% D n/a |

-
-
756-18-3
bis-heptafluoropropyl-iodo-phosphine

-
A
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
B
-
678-07-9
heptafluoropropyl-diiodo-phosphine

| Conditions | Yield |
|---|---|
| 220°C (48 h); | A 16% B 2% |
| at 220℃; |
-
-
116-14-3
polytetrafluoroethylene

-
-
2314-97-8
iodotrifluoromethane

-
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

| Conditions | Yield |
|---|---|
| Irradiation.mit UV-Licht; | |
| photochemische Bildung; |
-
-
116-14-3
polytetrafluoroethylene

-
-
2314-97-8
iodotrifluoromethane

-
A
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
B
-
638-79-9
undecafluoro-1-iodopentane

| Conditions | Yield |
|---|---|
| UV.Irradiation; |


| Conditions | Yield |
|---|---|
| With iodine at 550 - 600℃; in der Dampfphase; |

| Conditions | Yield |
|---|---|
| at 100℃; |
-
-
678-07-9
heptafluoropropyl-diiodo-phosphine

-
A
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
B
-
756-18-3
bis-heptafluoropropyl-iodo-phosphine

| Conditions | Yield |
|---|---|
| at 220℃; |

| Conditions | Yield |
|---|---|
| With iodine at 100℃; | |
| With air; iodine at 150 - 160℃; | |
| With Perfluorotributylamine; iodine at 115℃; |

| Conditions | Yield |
|---|---|
| With iodine In gas at 560 - 700℃; under 30 - 300 Torr; Equilibrium constant; |
-
-
116-15-4
perfluoropropylene

-
-
2314-97-8
iodotrifluoromethane

-
A
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
B
-
382-21-8
perfluoroisobutylene

-
C
-
677-69-0
perfluoroisopropyl iodide

-
D
-
1542-18-3
2-trifluoromethyl-perfluoropropyl iodide

-
E
-
4459-18-1
perfluoro-tert-butyl iodide

| Conditions | Yield |
|---|---|
| at 360℃; Product distribution; nickel reactor; other temperatures, influence of reactor material; |

-
-
107432-46-2
Perfluoro(1-propoxyethyl) iodide

-
A
-
354-34-7
trifluoroacetyl fluoride

-
B
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

| Conditions | Yield |
|---|---|
| at 250℃; under 7500.6 Torr; Pyrex tube; |
-
-
80392-00-3
C8H19O2P*C3F7I

-
A
-
76509-66-5
diisopropyl ethylphosphonite

-
B
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

| Conditions | Yield |
|---|---|
| In octane at 19.9℃; Equilibrium constant; other temperatures; ΔH; ΔS; ΔG; |
-
-
80391-99-7
C8H19O2P*C3F7I

-
A
-
20355-93-5
dipropyl ethylphosphonite

-
B
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

| Conditions | Yield |
|---|---|
| In octane at 19.9℃; Equilibrium constant; other temperatures; ΔH; ΔS; ΔG; |
-
-
80392-02-5
C10H23O2P*C3F7I

-
A
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
B
-
24681-02-5
Diisobutoxy-ethylphosphin

| Conditions | Yield |
|---|---|
| In octane at 19.9℃; Equilibrium constant; other temperatures; ΔH; ΔS; ΔG; |
-
-
80392-01-4
C10H23O2P*C3F7I

-
A
-
24603-81-4
dibutyl ethylphosphonite

-
B
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

| Conditions | Yield |
|---|---|
| In octane at 19.9℃; Equilibrium constant; other temperatures; ΔH; ΔS; ΔG; |
-
-
26735-60-4
heptafluoropropyl-difluoro-λ3-iodane

-
-
2170-06-1
1-Phenyl-2-(trimethylsilyl)acetylene

-
A
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
B
-
932-88-7
1-iodo-2-phenylethyne

-
C
-
886-66-8
1,4-diphenyl-1,3-butadiyne

-
D
-
81674-08-0
3,3,4,4,5,5,5-heptafluoro-1-phenyl-1-pentyne

| Conditions | Yield |
|---|---|
| With potassium fluoride; 18-crown-6 ether In dichloromethane at 20℃; for 0.5h; |

-
-
375-22-4
heptafluorobutyric Acid

-
-
7553-56-2
iodine

-
A
-
116-15-4
perfluoropropylene

-
B
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

| Conditions | Yield |
|---|---|
| at 260℃; Reaktion des Natrium-Salzes; |

-
-
375-18-8
heptafluoro-butyryl iodide

-
-
7553-56-2
iodine

-
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

| Conditions | Yield |
|---|---|
| at 600℃; |

| Conditions | Yield |
|---|---|
| With iodine In gas at 560 - 700℃; under 30 - 300 Torr; Equilibrium constant; |
-
-
116-14-3
polytetrafluoroethylene

-
-
2314-97-8
iodotrifluoromethane

-
A
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

| Conditions | Yield |
|---|---|
| im UV-Licht.Irradiation; | |
| at 220℃; |

-
-
116-14-3
polytetrafluoroethylene

-
-
2314-97-8
iodotrifluoromethane

-
A
-
354-64-3
Pentafluoroethyl iodide

-
B
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
C
-
423-39-2
1-iodo-2,2,3,3,4,4,5,5,5-nonafluorobutane

-
D
-
638-79-9
undecafluoro-1-iodopentane

-
E
-
355-43-1
n-perfluorohexyl iodide

-
F
-
335-58-0
perfluoroheptyl iodide

| Conditions | Yield |
|---|---|
| In gas Product distribution; Quantum yield; Thermodynamic data; Irradiation; pulsed CO2 laser; release of reaction enthalpy, selectivity depending on pressure and temperature; |

-
-
116-14-3
polytetrafluoroethylene

-
-
2314-97-8
iodotrifluoromethane

-
A
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
B
-
677-69-0
perfluoroisopropyl iodide

| Conditions | Yield |
|---|---|
| With aluminum chlorofluoride at 25 - 30℃; for 16h; Addition; Title compound not separated from byproducts; |

-
-
1623-05-8
perfluoro(propyl vinyl ether)

-
-
1184-76-5
difluorodiiodomethane

-
A
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
B
-
91095-98-6
2,2,3,3-Tetrafluoro-3-iodo-propionyl fluoride

| Conditions | Yield |
|---|---|
| at 185℃; for 3.5h; Title compound not separated from byproducts; |

-
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
-
1071001-11-0
1,1,1,2,2,3,3-heptafluoro-8-phenyloctan-4-one

| Conditions | Yield |
|---|---|
| Stage #1: 1,1,1,2,2,3,3-heptafluoro-3-iodo-propane; 5-phenylpentanoic acid-methoxy-methyl-amide With methyllithium lithium bromide In diethyl ether at -78℃; for 1.5h; Stage #2: With potassium hydrogensulfate In diethyl ether; water pH=5.0; | 99% |
| With methyllithium lithium bromide In diethyl ether at -78℃; for 1.5h; Product distribution / selectivity; | 99% |
-
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
-
2097-18-9
1-vinylsilatrane

-
-
112330-85-5
1-(2-perfluoropropyl-1-iodoethyl)silatrane

| Conditions | Yield |
|---|---|
| In chloroform for 3h; Irradiation; | 98.1% |
| In chloroform for 3h; Irradiation; | 98% |
-
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
-
407-25-0
trifluoroacetic anhydride

-
-
77758-71-5
heptafluoropropyliodine bis(trifluoroacetate)

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In trifluoroacetic acid for 17h; Ambient temperature; | 98% |
| With dihydrogen peroxide | |
| With dihydrogen peroxide; trifluoroacetic acid |
-
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
-
118364-67-3
4-methoxycarbonylaminothiophenol

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 21 - 22℃; for 0.5h; | 98% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; for 6h; Irradiation; | 98% |
-
-
84692-66-0
CH2CHCH2Ge(O(CH2)2)3N

-
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
-
70565-61-6
1-iodogermatrane

| Conditions | Yield |
|---|---|
| In dichloromethane Irradiation (UV/VIS); mixt. irradiation (5 min, 20°C, vac.); (1)H-NMR spectroscopy; | 97% |
-
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
-
66-77-3
1-naphthaldehyde

-
-
62509-75-5
2,2,3,3,4,4,4-heptafluoro-1-(1-naphthyl)-1-hydroxybutane

| Conditions | Yield |
|---|---|
| Stage #1: 1,1,1,2,2,3,3-heptafluoro-3-iodo-propane With zirconocene dichloride; butyl magnesium bromide In 1,4-dioxane; diethyl ether at -78℃; for 1h; Inert atmosphere; Stage #2: 1-naphthaldehyde In 1,4-dioxane; diethyl ether at 20℃; for 1h; Inert atmosphere; chemoselective reaction; | 97% |
| Stage #1: 1,1,1,2,2,3,3-heptafluoro-3-iodo-propane With zirconocene dichloride; 1-propylmagnesium chloride In 1,4-dioxane; diethyl ether at -78 - 0℃; for 1h; Stage #2: 1-naphthaldehyde In 1,4-dioxane; diethyl ether at 20℃; for 2h; Reagent/catalyst; Temperature; | 97% |

-
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

| Conditions | Yield |
|---|---|
| With ammonium cerium (IV) nitrate; 2,2'-azobis(isobutyronitrile); silver trifluoroacetate; 2-iodo-1-methoxyethane; 2,3-butane diamine; N-fluorobis(benzenesulfon)imide at 90℃; for 5h; Reagent/catalyst; | 96.5% |
| With 2,2'-azobis(isobutyronitrile); di-tert-butyl peroxide In acetonitrile at 105℃; for 12h; | 75% |
-
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
-
4109-84-6
trichlorovinylstannane

-
-
170308-93-7
bis(perfluoropropyl)vinyltin chloride

| Conditions | Yield |
|---|---|
| With isopropylmagnesium chloride In diethyl ether byproducts: i-PrI; Ar-atmosphere; treatment of C3F7I with i-PrMgCl, slow addn. of Sn-compd.(-70°C), stirring at -40°C for 2 h, warming to room temp. overnight; distn. (reduced pressure); elem. anal.; | 96% |
-
-
592-41-6
1-hexene

-
-
6479-18-1
1-methyl-2(1H)-quinoxalinone

-
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In 1-methyl-pyrrolidin-2-one at 25℃; for 16h; Schlenk technique; Inert atmosphere; Sealed tube; Irradiation; | 96% |


| Conditions | Yield |
|---|---|
| at 25 - 30℃; for 6h; Irradiation; | 95% |

| Conditions | Yield |
|---|---|
| In hexane byproducts: C2H5I, ZnI2; dry N2 atm.; cooling (-78°C), stirring (-20°C, 3 h), heating (room temp., 15 min); evapn. (vac.); elem. anal.; | 95% |

-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

-
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
-
557-20-0
diethylzinc

| Conditions | Yield |
|---|---|
| In hexane at -60 - -20℃; for 48h; Inert atmosphere; | 95% |
| In hexane; toluene at -35 - -5℃; for 24h; Inert atmosphere; | 93% |
| In hexane; toluene at -41 - -5℃; for 12h; Inert atmosphere; | 92% |

-
-
111-66-0
oct-1-ene

-
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
-
23885-16-7
1,1,1,2,2,3,3-heptafluoro-5-iodoundecane

| Conditions | Yield |
|---|---|
| With silver(I) acetate; tin(ll) chloride In methanol Ambient temperature; | 94% |
| With ethanolamine; copper(l) chloride; copper dichloride In tert-butyl alcohol for 24h; Heating; |
-
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
A
-
105062-53-1
C3F17IO2Te2

| Conditions | Yield |
|---|---|
| With ClOTeF5 at 25℃; for 72h; | A 94% B n/a |
-
-
4383-22-6
Allylbenzylamine

-
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
-
124-38-9
carbon dioxide

| Conditions | Yield |
|---|---|
| Stage #1: Allylbenzylamine; carbon dioxide With 1,3,4,6,7,8-hexahydro-2H-pyrimido[1,2-a]pyrimidine In acetonitrile at 20℃; for 2h; Autoclave; Stage #2: 1,1,1,2,2,3,3-heptafluoro-3-iodo-propane In acetonitrile at 20℃; under 750.075 Torr; for 12h; Autoclave; Irradiation; | 94% |


| Conditions | Yield |
|---|---|
| With 2,4,6-trimethyl-pyridine; (4,4'-di-tert-butyl-2,2'-dipyridyl)-bis-(2-phenylpyridine(-1H))-iridium(III) hexafluorophosphate; ascorbic acid In dimethyl sulfoxide at 23 - 25℃; for 2h; Inert atmosphere; Irradiation; | 94% |
-
-
7440-36-0
antimony

-
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
-
756-18-3
bis-heptafluoropropyl-iodo-phosphine

-
A
-
51761-69-4
Tris(heptafluor-n-propyl)phosphan

-
B
-
7790-44-5
antimony triiodide

| Conditions | Yield |
|---|---|
| 230°C (16 h); | A 93% B n/a |
| 230°C (16 h); | A 93% B n/a |

-
-
59645-28-2
1,2-bis(heptafluoropropyl)-1,2-bis(trifluoromethyl)diphosphine

-
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
A
-
20608-32-6
Trifluormethyl-bis(heptafluor-n-propyl)phosphan

-
B
-
51577-24-3
trifluoromethyl(heptafluoro-n-propyl)iodophosphine

| Conditions | Yield |
|---|---|
| Irradiation (UV/VIS); 16°C, 1 h; | A 93% B n/a |
| Irradiation (UV/VIS); 16°C, 1 h; | A 93% B n/a |
| 167°C in dark; |

-
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
-
14243-78-8
Ir(CO)(PPh3)(2,4-pentanedionato)

-
A
-
1118766-38-3
Ir(CO)(PPh3)(2,4-pentanedionato)(CF2CF2CF3)(I)

| Conditions | Yield |
|---|---|
| In dichloromethane (N2) to soln. Ir complex in CH2Cl2 n-C3F7I was added at 0°C; solvent was removed in vacuo, residue was recrystd. from hexane-ether; elem. anal.; | A n/a B 93% |

-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

-
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
-
557-20-0
diethylzinc

| Conditions | Yield |
|---|---|
| In hexane; toluene at -35 - -5℃; for 24h; Inert atmosphere; | 93% |

-
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
-
41795-26-0
benzoic acid hex-5-enyl ester

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene; tri-(9-anthryl)borane In acetonitrile at 20℃; for 6h; Inert atmosphere; Irradiation; | 93% |
-
-
627-27-0
homoalylic alcohol

-
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
-
89621-93-2
1H,1H,2H,2H,3H,4H,4H-heptafluoro-3-iodohepta-1-ol

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0) In hexane at 4 - 20℃; for 2h; Inert atmosphere; | 92% |
| With tetrakis(triphenylphosphine) palladium(0) In hexane at 4 - 20℃; for 2h; Inert atmosphere; | 92% |
| tetrakis(triphenylphosphine) palladium(0) In hexane Ambient temperature; | 65% |
| With pyridine; triiron dodecarbonyl at 60℃; for 2h; | 85 % Chromat. |
-
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
-
593-91-9
trimethylbismuthine

-
A
-
2138-73-0
Dimethyl-heptafluorpropyl-wismut

-
B
-
1957-54-6
Methyl-bis-heptafluorpropyl-wismut

| Conditions | Yield |
|---|---|
| 100°C (12 h); | A 92% B 8% |
| 100°C (12 h); | A 92% B 8% |

-
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
-
1146964-22-8
3,3-diallyl-1,5-dioxa-spiro[5.5]undecane-2,4-dione

-
-
1146964-23-9
C18H20F7IO4

| Conditions | Yield |
|---|---|
| With sodium dithionite; sodium carbonate In water; acetonitrile at 0℃; for 3h; | 92% |
-
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

| Conditions | Yield |
|---|---|
| In dichloromethane at 35℃; for 120h; Darkness; | 92% |

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene; tri-(9-anthryl)borane In acetonitrile at 20℃; for 6h; Inert atmosphere; Irradiation; | 92% |

| Conditions | Yield |
|---|---|
| With pyrrolidine; Diphenylacetaldehyde In dichloromethane at 20℃; for 16h; Inert atmosphere; Irradiation; | 92% |
-
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
-
141-43-5
ethanolamine

-
-
754-05-2
ethenyltrimethylsilane

-
-
89608-32-2
(1-iodo-3,3,4,4,5,5,5-heptafluoropentyl)trimethylsilane

| Conditions | Yield |
|---|---|
| In ethanol | 91% |
| copper(I) chloride | 2% |
| With iron(III) chloride |

Heptafluoro-1-iodopropane Specification
The Propane,1,1,1,2,2,3,3-heptafluoro-3-iodo- with CAS registry number of 754-34-7 is also called Perfluoropropyl iodide and n-Heptafluoropropyl iodide. It belongs to categories of Organic Fluorides. Both systematic name and IUPAC name are the same which is called 1,1,1,2,2,3,3-heptafluoro-3-iodopropane. Its EINECS registry number is 212-045-5.
Physical properties about this chemical are: (1) ACD/LogP: 4.31; (2) ACD/LogD (pH 5.5): 4.31; (3) ACD/LogD (pH 7.4): 4.31; (4) ACD/BCF (pH 5.5): 1107.2; (5) ACD/BCF (pH 7.4): 1107.2; (6) ACD/KOC (pH 5.5): 5256.01; (7) ACD/KOC (pH 7.4): 5256.01; (8) #Freely Rotating Bonds: 1; (9) Index of Refraction: 1.361; (10) Molar Refractivity: 29.96 cm3; (11) Molar Volume: 135.1 cm3 ; (12) Polarizability: 11.87×10-24 cm3; (13) Surface Tension: 17.7 dyne/cm; (14) Density: 2.189 g/cm3; (15) Flash Point: 3.4 °C; (16) Enthalpy of Vaporization: 27.09 kJ/mol; (17) Boiling Point: 37.9 °C at 760 mmHg; (18) Vapour Pressure: 478 mmHg at 25°C; (19) Melting Point: -95 °C; (20) Storage temp. : 2-8 °C; (21) Sensitive: Light Sensitive; (22) Stability: Stable. Incompatible with strong oxidizing agents.
Preparation of Propane,1,1,1,2,2,3,3-heptafluoro-3-iodo- : this chemical is prepared by C3F17IO2Te2.
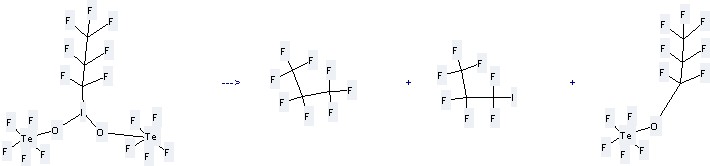
This reaction needs the temperature of 115 °C, and the reaction time is 26 hours. The yield is 30%.
Uses of Propane,1,1,1,2,2,3,3-heptafluoro-3-iodo-: it is used to produce other chemicals. For example, it is used to produce 1H,2H-heptafluoro-1-iodo-pent-1-ene.
.jpg)
This reaction will occur at temperature of 200 °C for 6 hours and other condition of steel cylinder. The yield is 76%.
When you are using this chemical, please be cautious about it as the following:
As a chemical, it is irritating to eyes, respiratory system and skin. Avoid contact with skin and eyes. And do not breathe vapour.
You can still convert the following datas into molecular structure:
(1) SMILES: FC(F)(F)C(F)(F)C(F)(F)ICopyCopied;
(2) InChI: InChI=1/C3F7I/c4-1(5,2(6,7)8)3(9,10)11;
(3) InChIKey: XTGYEAXBNRVNQU-UHFFFAOYAV;
(4) Std. InChI: InChI=1S/C3F7I/c4-1(5,2(6,7)8)3(9,10)11;
(5) Std. InChIKey: XTGYEAXBNRVNQU-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
| mouse | LC50 | inhalation | 404gm/m3/2H (404000mg/m3) | "Toxicometric Parameters of Industrial Toxic Chemicals Under Single Exposure," Izmerov, N.F., et al., Moscow, Centre of International Projects, GKNT, 1982Vol. -, Pg. 76, 1982. |
Related Products
- Heptafluoro-1-iodopropane
- 75434-70-7
- 7543-51-3
- 75436-40-7
- 75438-54-9
- 75438-57-2
- 75438-58-3
- 75444-69-8
- 75-44-5
- 7544-75-4
- 7545-23-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View