-
Name
1-Hexanol
- EINECS 203-852-3
- CAS No. 111-27-3
- Article Data637
- CAS DataBase
- Density 0.817 g/cm3
- Solubility 6 g/L (25 ºC)
- Melting Point -52 °C(lit.)
- Formula C6H14O
- Boiling Point 158.153 °C at 760 mmHg
- Molecular Weight 102.177
- Flash Point 60 °C
- Transport Information UN 2282 3/PG 3
- Appearance Colourless liquid
- Safety 24/25
- Risk Codes 22
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Epal 6;NSC 9254;Pentylcarbinol;n-Hexan-1-ol;n-Hexanol;n-Hexylalcohol;Hexylalcohol (8CI);1-Hexyl alcohol;1-Hydroxyhexane;Amylcarbinol;Caproyl alcohol;1-Hexanol;
- PSA 20.23000
- LogP 1.55900
Synthetic route

| Conditions | Yield |
|---|---|
| With sodium aluminum tetrahydride In tetrahydrofuran at 0℃; for 0.5h; | 100% |
| With C30H34Cl2N2P2Ru; potassium methanolate; hydrogen In tetrahydrofuran at 100℃; under 38002.6 - 76005.1 Torr; for 15h; Glovebox; Autoclave; | 93% |
| With ethanol; ruthenium(bis[2‐(ethylsulfanyl)ethyl]amine)(dichloro)(triphenylphosphine); potassium tert-butylate In toluene at 80℃; for 16h; Catalytic behavior; Reagent/catalyst; | 89% |

| Conditions | Yield |
|---|---|
| With Na-phosphate buffer; horse liver NADH; NAD; sodium formate; <(C5Me5)Rh(bpy-5-OAc)(H2O)>Cl2 at 25℃; for 30h; Product distribution; Mechanism; other ketones, other times, other temperatures, other enzymes; | 100% |
| With sodium aluminum tetrahydride In tetrahydrofuran at 0℃; for 0.0833333h; Product distribution; other aldehydes, ketones, carboxylic acids (also sodium salts), acid chlorides, esters, lactones, epoxides, amides, nitriles, nitrogen and sulfur compounds; var. temp., time and ratio of reagents; | 100% |
| With sodium aluminum tetrahydride In tetrahydrofuran at 0℃; for 0.0833333h; | 100% |

| Conditions | Yield |
|---|---|
| With C39H39N6ORu(1+)*Br(1-); potassium methanolate; hydrogen In tetrahydrofuran at 70℃; under 37503.8 Torr; for 4h; Reagent/catalyst; | 100% |
| With C56H70Cl3N10Ru2(1+)*F6P(1-); potassium tert-butylate; hydrogen In tetrahydrofuran; dodecane at 70℃; under 37503.8 Torr; for 16h; Inert atmosphere; Glovebox; Autoclave; | 100% |
| With C30H26Cl2N3PRu; hydrogen; sodium ethanolate In toluene at 80℃; under 38002.6 Torr; for 16h; Catalytic behavior; Autoclave; Inert atmosphere; Schlenk technique; | 95% |
-
-
82965-03-5
2,4,6-triisopropyl-benzenesulfonic acid n-hexylester

-
A
-
717-74-8
1,3,5-triisopropyl benzene

-
B
-
111-27-3
hexan-1-ol

| Conditions | Yield |
|---|---|
| With lithium amalgam In N,N-dimethyl-formamide; toluene Product distribution; Mechanism; further solvents; | A 100% B 100% |

| Conditions | Yield |
|---|---|
| With C56H70Cl3N10Ru2(1+)*F6P(1-); potassium tert-butylate; hydrogen In tetrahydrofuran; dodecane at 70℃; under 37503.8 Torr; for 16h; Inert atmosphere; Glovebox; Autoclave; | 100% |
| With C66H102N4OP2Ru; hydrogen In toluene at 105℃; under 22502.3 Torr; for 20h; Inert atmosphere; Glovebox; | 99% |
| With C31H26ClN2OPRu; hydrogen; sodium methylate In tetrahydrofuran at 80℃; for 2h; | 98% |

| Conditions | Yield |
|---|---|
| With C56H70Cl3N10Ru2(1+)*F6P(1-); potassium tert-butylate; hydrogen In tetrahydrofuran; dodecane at 70℃; under 37503.8 Torr; for 16h; Inert atmosphere; Glovebox; Autoclave; | 100% |

| Conditions | Yield |
|---|---|
| silica-supported prop-1-ylsulfonic acid In methanol | 99.6% |
| With silica gel; iron(III) chloride for 45h; Ambient temperature; | 98.5% |
| ammonium cerium(IV) nitrate In alkaline aq. solution; acetonitrile at 70℃; for 2h; pH=8; Decomposition; | 94% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-hexene With 9-bora-bicyclo[3.3.1]nonane Stage #2: With sodium peroxoborate tetrahydrate | 99% |
| Stage #1: 1-hexene With borane-THF In tetrahydrofuran at 25℃; for 0.0138889h; Flow reactor; Stage #2: With dihydrogen peroxide; sodium hydroxide In tetrahydrofuran; ethanol; water at 20℃; for 0.00555556h; Flow reactor; | 88% |
| With di-n-pentylbromoborane; alkaline H2O2; sodium hydride 1.) diglyme, room temperature, 7 h; further dialkylbromoboranes; Yield given. Multistep reaction; |
-
-
82965-02-4
hexyl 2,4,6-trimethylbenzenesulfonate

-
A
-
108-67-8
1,3,5-trimethyl-benzene

-
B
-
111-27-3
hexan-1-ol

| Conditions | Yield |
|---|---|
| With lithium amalgam In N,N-dimethyl-formamide; toluene Product distribution; Mechanism; further solvents; | A 85% B 99% |

| Conditions | Yield |
|---|---|
| With hydrogen; copper-palladium; silica gel In ethanol at 25℃; under 760 Torr; Kinetics; | A 99% B n/a |
| With hydrogen In methanol at 30℃; under 760.051 Torr; for 3h; | A 99% B 1% |
| With hydrogen In methanol at 20℃; under 150.015 - 900.09 Torr; for 0.0116667h; Inert atmosphere; Schlenk technique; Green chemistry; | A n/a B n/a |

| Conditions | Yield |
|---|---|
| With water In aq. phosphate buffer at 20℃; pH=7.4; Electrolysis; Inert atmosphere; Enzymatic reaction; | 98.3% |
| With hydrogen; Rh/Al2O3; molybdenum hexacarbonyl In 1,2-dimethoxyethane at 150℃; under 76000 Torr; for 16h; | 95% |
| With 1,1,3,3-Tetramethyldisiloxane; copper(II) bis(trifluoromethanesulfonate) In toluene at 80℃; for 16h; sealed tube; | 94% |

| Conditions | Yield |
|---|---|
| With C24H20ClN2OPRu; potassium tert-butylate; hydrogen In tetrahydrofuran at 110℃; under 10640.7 Torr; for 36h; Inert atmosphere; Schlenk technique; | 98% |
| With ethanol; sodium | |
| With sodium aluminum tetrahydride In tetrahydrofuran for 12h; Ambient temperature; |

| Conditions | Yield |
|---|---|
| With lithium amalgam In 1,4-dioxane; isopropyl alcohol at 23℃; for 2h; | A 98% B 98% |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; palladium diacetate In methanol at 20℃; for 0.5h; | 98% |

| Conditions | Yield |
|---|---|
| With hydrogen In tetrahydrofuran at 30℃; under 760.051 Torr; for 6h; | A 98% B 2% |

| Conditions | Yield |
|---|---|
| In sodium hydroxide; water | 97% |
-
-
74058-62-1
1-(2-Thioxo-thiazolidin-3-yl)-hexan-1-one

-
-
111-27-3
hexan-1-ol

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; water In tetrahydrofuran Ambient temperature; | 96% |
-
-
781-07-7
benzenesulfonic acid n-hexylester

-
A
-
16883-74-2
lithium benzenesulfinate

-
B
-
111-27-3
hexan-1-ol

| Conditions | Yield |
|---|---|
| With lithium amalgam In N,N-dimethyl-formamide; toluene at 23℃; for 2h; Product distribution; Mechanism; further solvents (effect of pKa); | A 95% B 95% |
-
-
3839-35-8
4-methylbenzenesulfonic acid n-hexylester

-
A
-
536-57-2
p-toluene sulfinic acid

-
B
-
111-27-3
hexan-1-ol

| Conditions | Yield |
|---|---|
| With lithium amalgam In 1,4-dioxane; isopropyl alcohol at 23℃; for 2h; | A 95% B 95% |
-
-
69564-60-9
4-chlorobenzenesulfonic acid n-hexylester

-
A
-
100-03-8
4-chlorobenzenesulfinic acid

-
B
-
111-27-3
hexan-1-ol

| Conditions | Yield |
|---|---|
| With lithium amalgam In 1,4-dioxane; isopropyl alcohol at 23℃; for 2h; | A 91% B 95% |

| Conditions | Yield |
|---|---|
| With aniline; (ϖ-allyl)palladium triflate based catalyst at 50℃; for 2h; | 94% |
| With lithium aluminium tetrahydride; bis(cyclopentadienyl)titanium dichloride In tetrahydrofuran for 8h; Ambient temperature; | 84% |
| tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride; polydimethylsiloxane In methanol; water at 50℃; for 24h; | 77% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-hexen-1-ol With lithium triethylborohydride; cobalt(II) bromide In tetrahydrofuran Inert atmosphere; Glovebox; Stage #2: With hydrogen In tetrahydrofuran at 60℃; under 7500.75 Torr; for 3h; | 94% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-hexene With Thexylboran Stage #2: With sodium peroxoborate tetrahydrate | A n/a B 93% |
| With borane-THF; dihydrogen peroxide; sodium carbonate 1.) THF, 0 deg C; 2.) H2O/THF, 50 deg C, 1 h; Yield given. Multistep reaction. Yields of byproduct given; | |
| With dimesitylboron hydride In tetrahydrofuran at 25℃; for 8h; Yields of byproduct given. Title compound not separated from byproducts; | A n/a B 96 % Chromat. |
-
-
69564-56-3
4-methoxybenzenesulfonic acid n-hexylester

-
A
-
1709-60-0
4-methoxybenzenesulfinic acid

-
B
-
111-27-3
hexan-1-ol

| Conditions | Yield |
|---|---|
| With lithium amalgam In N,N-dimethyl-formamide at 23℃; for 2h; | A 93% B 90% |
-
-
80033-60-9
1-(tert-butyldimethylsilyl)oxyhexane

-
-
111-27-3
hexan-1-ol

| Conditions | Yield |
|---|---|
| With diisobutylaluminium hydride In dichloromethane; toluene at 23℃; for 2h; | 93% |

| Conditions | Yield |
|---|---|
| With carbonylchloro[4,5-bis(diisopropylphosphinomethyl)acridine]hydridoruthenium(II); water; hydrogen In 1,4-dioxane at 135℃; under 3750.38 Torr; for 48h; Pressure; Schlenk technique; Inert atmosphere; | 93% |
-
-
111-28-4
2,4-hexadiene-1-ol

-
A
-
928-96-1
(Z)-3-Hexen-1-ol

-
B
-
928-97-2
(E)-3-hexen-1-ol

-
C
-
6126-50-7, 928-91-6, 928-92-7
hex-4-en-1-ol

-
D
-
111-27-3
hexan-1-ol

| Conditions | Yield |
|---|---|
| With hydrogen; (methyl benzoate)Cr(CO)6 In methanol at 190 - 200℃; under 36775.4 Torr; for 2h; | A 92.5% B 4.6% C 2.3% D 0.6% |
| With hydrogen; (1,2,4,5-tetramethylbenzene)tricarbonylchromium(0) In methanol at 190 - 200℃; under 36775.4 Torr; for 4h; Product distribution; other arene ligands, other solvents; also in absence of arene ligands; |


| Conditions | Yield |
|---|---|
| With hydrogen In 1,2-dimethoxyethane at 80℃; under 60006 Torr; for 2h; Reagent/catalyst; Pressure; Solvent; Temperature; | A 92.3% B 8% |
| With hydrogen; (acetylacetonato)dicarbonylrhodium (l); molybdenum hexacarbonyl In 1,4-dioxane at 150℃; under 75007.5 Torr; for 3h; | A 79% B 7% |

| Conditions | Yield |
|---|---|
| With Na12(Ga4(1,5-bis(2,3-dihydroxybenzamido)naphthalene))6; [(DMPE)Rh(COD)][BF4]; hydrogen In water-d2 at 20℃; for 12h; Reagent/catalyst; | 92% |

| Conditions | Yield |
|---|---|
| at 20 - 50℃; for 1h; Temperature; | 100% |
| at 100 - 110℃; | |
| at 20 - 135℃; |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at -15℃; for 1h; Green chemistry; | 100% |
| With triethylamine | 99% |
| With triethylamine In dichloromethane at 0 - 20℃; | 99% |

| Conditions | Yield |
|---|---|
| With C19H35Cl2CoN2P; sodium triethylborohydride In toluene at 150℃; for 24h; | 100% |
| With chloro(η5-pentamethylcyclopentadienyl)(L-prolinato)iridium(III) In toluene at 95℃; for 24h; Inert atmosphere; Sealed tube; | 98% |
| With chloro(η5-pentamethylcyclopentadienyl)(L-prolinato)iridium(III) In toluene at 95℃; for 24h; | 98% |

| Conditions | Yield |
|---|---|
| With dilithium tetra(tert-butyl)zincate In toluene at 0℃; for 1h; Inert atmosphere; | 100% |
| With pseudomonas fuorescens lipase immobilized on multiwall carbon nano-tubes at 50℃; for 5h; Green chemistry; | 99% |
| With sulfuric acid beim Erhitzen; | |
| With aluminium trichloride at 105℃; | |
| In diethyl ether at 35℃; Candida cylindracea lipase; |

| Conditions | Yield |
|---|---|
| With C23H42N2OP2Ru for 12h; Reflux; Inert atmosphere; Darkness; | 100% |
| Ru complex at 157℃; for 24h; Inert atmosphere; | 99% |
| With calcium hypochlorite In water; acetic acid; acetonitrile at 0℃; for 1h; | 98% |

| Conditions | Yield |
|---|---|
| With 4 A molecular sieve; tetrabutylammonium periodite; sodium ruthenate(VI) In dichloromethane at 20℃; for 24h; Oxidation; | 100% |
| With 4 A molecular sieve; tetrabutylammonium perchlorate; Ru-Cu-Al-hydrotalcite In toluene at 60℃; for 24h; | 100% |
| With iodosylbenzene; Cl-CH2-PS supported 5-amino-1,10-phenanthroline-Ru In acetonitrile at 60℃; for 2h; | 100% |

| Conditions | Yield |
|---|---|
| With air; potassium carbonate In water at 66.84℃; for 24h; | 100% |
| With oxygen In water at 80℃; under 760.051 Torr; for 24h; | 99.3% |
| Stage #1: hexan-1-ol With gold on titanium oxide In water at 90℃; for 0.166667h; Inert atmosphere; Stage #2: With dihydrogen peroxide In water at 90℃; for 1.08333h; Inert atmosphere; chemoselective reaction; | 99% |
-
-
148160-26-3
3-tert-butoxycarbonyl-2-chloro-1,3,2-oxazaphospholidine

-
-
111-27-3
hexan-1-ol

-
-
148160-27-4
3-tert-butoxycarbonyl-2-hexyloxy-1,3,2-oxazaphospholidine

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane -60 deg C to r.t., 1 h; | 100% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In benzene | 100% |
| toluene-4-sulfonic acid In toluene for 18h; Heating / reflux; | 96% |
| With toluene-4-sulfonic acid | 84% |
| With toluene-4-sulfonic acid In benzene for 16h; Heating; | 84% |

-
-
111-27-3
hexan-1-ol

| Conditions | Yield |
|---|---|
| With polyvinylpyridine polymer-supported dimethylaminopyridine; polystyrene-bound N-cyclohexylcarbodiimide In dichloromethane at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| With triphenylphosphine; diethylazodicarboxylate In tetrahydrofuran; toluene at 0 - 20℃; for 0.5h; Inert atmosphere; | 100% |
| With triphenylphosphine; diethylazodicarboxylate In tetrahydrofuran at 20℃; for 1h; | 95% |

| Conditions | Yield |
|---|---|
| With triethylsilane; t-Bu-P4 In hexane; dimethyl sulfoxide at 100℃; for 2h; | 100% |

| Conditions | Yield |
|---|---|
| at 85℃; for 24h; | 100% |
-
-
286371-46-8
4-[(6,7-dimethoxy-4-quinolyl)oxy]-2,5-dimethylaniline

-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
144-55-8
sodium hydrogencarbonate

-
-
111-27-3
hexan-1-ol

| Conditions | Yield |
|---|---|
| With triethylamine In methanol; dichloromethane; chloroform; toluene | 100% |


| Conditions | Yield |
|---|---|
| With copper(II) bis(trifluoromethanesulfonate) at 100℃; for 0.0833333h; Microwave irradiation; | 100% |

| Conditions | Yield |
|---|---|
| With oxidase In water at 40℃; for 1.5h; Reformatsky Reaction; | 100% |
| With oxygen In neat (no solvent) at 45℃; for 7h; Solvent; Temperature; | 50 %Chromat. |

| Conditions | Yield |
|---|---|
| With bismuth(lll) trifluoromethanesulfonate In 1,2-dichloro-ethane at 80℃; for 24h; Reagent/catalyst; Inert atmosphere; Schlenk technique; chemoselective reaction; | 100% |
| With toluene-4-sulfonic acid In neat (no solvent) at 120℃; for 24h; Inert atmosphere; Sealed tube; | 98% |
| With p-toluene sulfonic acid - choline chloride covalently immobilized on polymeric microspheres coated iron oxide magnetic nanoparticles In neat (no solvent) at 120℃; for 12h; Reagent/catalyst; Solvent; chemoselective reaction; | 90% |

| Conditions | Yield |
|---|---|
| With bismuth(lll) trifluoromethanesulfonate In 1,2-dichloro-ethane at 80℃; for 24h; Inert atmosphere; Schlenk technique; chemoselective reaction; | 100% |

-
-
111-27-3
hexan-1-ol

| Conditions | Yield |
|---|---|
| With C28H27F6N3O3S In tetrahydrofuran at 25℃; for 72h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With nickel(II) sulfate hexahydrate; 4,4'-Dimethoxy-2,2'-bipyridin; C41H40O16; N-ethyl-N,N-diisopropylamine In acetonitrile at 20℃; for 24h; Schlenk technique; Irradiation; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| silica-supported prop-1-ylsulfonic acid In acetonitrile for 0.166667h; | 99.8% |
| With sulfated zirconia In dichloromethane for 1h; Ambient temperature; | 96% |
| With aminosulfonic acid at 15℃; for 4.5h; | 96% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide for 0.0194444h; microwave irradiation; | 99% |
| With H3[P(Mo3O10)4]*nH2O at 20℃; for 0.1h; | 97% |
| With zirconium phosphate In neat (no solvent) at 60℃; for 0.5h; Green chemistry; | 96% |

| Conditions | Yield |
|---|---|
| With C19H35Cl2CoN2P; sodium triethylborohydride In toluene at 150℃; for 24h; Catalytic behavior; Reagent/catalyst; Solvent; Temperature; Molecular sieve; Schlenk technique; | 99% |
| With C15H15ClN7Ru(1+)*Cl(1-); sodium tetraphenyl borate; 1,2-bis-(diphenylphosphino)ethane at 165℃; for 12h; Reagent/catalyst; Schlenk technique; Inert atmosphere; | 98% |
| With [Py(NP(iPr)2)(NHP(iPr)2)Ir(cod)]; potassium tert-butylate In diethylene glycol dimethyl ether at 110℃; for 24h; Inert atmosphere; | 95% |
Hexyl alcohol Specification
The Hexyl alcohol with CAS registry number of 111-27-3 is also called 1-Hydroxyhexane. The IUPAC name is hexan-1-ol. Its EINECS registry number is 203-852-3. In addition, the molecular formula is C6H14O and the molecular weight is 102.17. It belongs to the classes of Alkanols; Monofunctional & alpha,omega-Bifunctional Alkanes; Monofunctional Alkanes. And it is Colorless transparent liquid, with fruit fragrance, and slightly soluble in water, but miscible with ether and ethanol. What's more, it should be stored in a cool, ventilated and dry place.It is harmful if swallowed. You shoulad avoid contact with skin and eyes.
Physical properties about Hexyl alcohol are:
(1)ACD/LogP: 1.86; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.858; (4)ACD/LogD (pH 7.4): 1.858; (5)ACD/BCF (pH 5.5): 15.195; (6)ACD/BCF (pH 7.4): 15.195; (7)ACD/KOC (pH 5.5): 244.051; (8)ACD/KOC (pH 7.4): 244.051; (9)#H bond acceptors: 1; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 5; (12)Polar Surface Area: 20.23 Å2; (13)Index of Refraction: 1.416; (14)Molar Refractivity: 31.381 cm3; (15)Molar Volume: 125.082 cm3; (16)Polarizability: 12.44 ×10-24cm3; (17)Surface Tension: 27.947 dyne/cm; (18)Density: 0.817 g/cm3; (19)Flash Point: 60 °C; (20)Enthalpy of Vaporization: 44.5 kJ/mol; (21)Boiling Point: 158.153 °C at 760 mmHg; (22)Vapour Pressure: 0.947 mmHg at 25°C.
Preparation of Hexyl alcohol:
Hexyl alcohol can be prepared by magnesium butylbromide and epoxyethane. The magnesium butylbromide can be prepared by bromobutane and magnesium. In the presence of catalyst iodine, you can make the bromine butane solution react with magnesium flocks under stirring. The reaction process must be controlled backflowing and without water. And you should control the dropping speed of bromine butane solution. After finish dropping, access the epoxy ethane at the cooling condition and control the speed in order to maintain the reaction temperature at 10 °C.
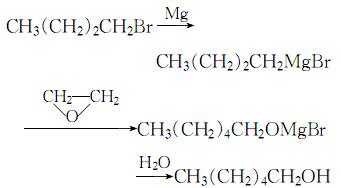
Uses of Hexyl alcohol:
Hexyl alcohol can be used in making preservatives and sleeping pills in medicine industry and manufacturing the plastic, synthetic lubricants, spices and medicine, etc. It also can be used as solvent of dye, all kinds of rubber, special printing ink, oil and natural resins. In addition, it can be used for analysis reagent and help solvent of nitric acid cellulose. Moreover, it can be used in baking goods, pudding and meat products.
You can still convert the following datas into molecular structure:
(1)SMILES: CCCCCCO;
(2)InChI: InChI=1/C6H14O/c1-2-3-4-5-6-7/h7H,2-6H2,1H3;
(3)InChIKey: ZSIAUFGUXNUGDI-UHFFFAOYAO.
The toxicity data of Hexyl alcohol is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LC | inhalation | > 1060ppm/6H (1060ppm) | Food and Cosmetics Toxicology. Vol. 13, Pg. 695, 1975. | |
| mammal (species unspecified) | LD50 | unreported | 2442mg/kg (2442mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 51(5), Pg. 61, 1986. | |
| mouse | LC | inhalation | > 1060ppm/6H (1060ppm) | Food and Cosmetics Toxicology. Vol. 13, Pg. 695, 1975. | |
| mouse | LD50 | intravenous | 103mg/kg (103mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 135, Pg. 330, 1962. | |
| mouse | LD50 | oral | 1950mg/kg (1950mg/kg) | Hygiene and Sanitation Vol. 31(1-3), Pg. 310, 1966. | |
| rabbit | LD50 | skin | 3100uL/kg (3.1mL/kg) | AMA Archives of Industrial Hygiene and Occupational Medicine. Vol. 4, Pg. 119, 1951. | |
| rat | LD | inhalation | > 1060ppm/6H (1060ppm) | Food and Cosmetics Toxicology. Vol. 13, Pg. 695, 1975. | |
| rat | LD50 | oral | 720mg/kg (720mg/kg) | LIVER: FATTY LIVER DEGERATION KIDNEY, URETER, AND BLADDER: OTHER CHANGES BLOOD: OTHER CHANGES | South African Medical Journal. Vol. 43, Pg. 795, 1969. |
Related Products
- Hexyl 2,2,2-trichloroacetate
- Hexyl 2,2-dimethylpropanoate
- Hexyl 2-methylbutyrate
- Hexyl 3-methylbutanoate
- Hexyl acetate
- Hexyl acrylate
- Hexyl alcohol
- Hexyl benzoate
- Hexyl beta-D-glucopyranoside
- Hexyl butyrate
- 111274-98-7
- 111-28-4
- 1112850-40-4
- 11128-96-4
- 11128-98-6
- 1112-91-0
- 11129-12-7
- 11129-15-0
- 11129-18-3
- 111291-97-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View