-
Name
L-tert-Leucine
- EINECS 200-522-0
- CAS No. 20859-02-3
- Article Data57
- CAS DataBase
- Density 1.038 g/cm3
- Solubility 125.5 g/L (20 °C) in water
- Melting Point 300 °C
- Formula C6H13NO2
- Boiling Point 217.7 °C at 760 mmHg
- Molecular Weight 131.175
- Flash Point 85.5 °C
- Transport Information
- Appearance white to almost white powder
- Safety 22-24/25
- Risk Codes
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms H-Tle-OH;L-tert-Leu-OH;L-tert·Leucine;L-Valine, 3-methyl-;L-tert Leucine;L-tert-Leucine(L-2-Amino-3,3-dimethylbutanoic acid);(s)-2-amino-3,3-dimethylbutanoic acid;
- PSA 63.32000
- LogP 1.14470
Synthetic route

-
A
-
20859-02-3
L-tert-Leucine

-
B
-
618-01-9
3,4-dimethylbenzenesulfonic acid

| Conditions | Yield |
|---|---|
| In water at 30 - 45℃; Product distribution / selectivity; | A 96.5% B 100% |

-
-
20859-02-3
L-tert-Leucine

| Conditions | Yield |
|---|---|
| With glutamate at 37℃; for 0.5h; pH=8; Kinetics; Reagent/catalyst; aq. phosphate buffer; Enzymatic reaction; optical yield given as %ee; | 100% |
-
-
319930-78-4
(R)-2-amino-3,3-dimethyl-butyric acid amide

-
-
71-36-3
butan-1-ol

-
-
20859-02-3
L-tert-Leucine

| Conditions | Yield |
|---|---|
| 98% |
-
-
62965-35-9
N-tert-butyloxycarbonyl-L-tert-leucine

-
-
20859-02-3
L-tert-Leucine

| Conditions | Yield |
|---|---|
| With hydrogenchloride In 1,4-dioxane | 97% |
| Stage #1: N-tert-butyloxycarbonyl-L-tert-leucine With methanesulfonic acid In dichloromethane at 25℃; for 3h; Stage #2: With triethylamine In dichloromethane at 25℃; for 1h; | 75% |
| With hydrogenchloride In water; toluene at 60℃; for 3h; |
-
-
202347-81-7
(3S,5S)-3-tert-Butyl-5-phenyl-morpholin-2-one

-
-
20859-02-3
L-tert-Leucine

| Conditions | Yield |
|---|---|
| With hydrogen; trifluoroacetic acid; palladium dihydroxide In methanol; water under 3750.3 Torr; for 24h; | 95% |

| Conditions | Yield |
|---|---|
| With pyridoxal 5'-phosphate; (S)-selective ω-transaminase from Paracoccus denitrificans; branched-chain aminoacid transaminase; 2-aminobutanoic acid In aq. phosphate buffer; hexane for 61h; pH=7; Kinetics; Solvent; Reagent/catalyst; Enzymatic reaction; enantioselective reaction; | A 94% B n/a |

-
-
319930-78-4
(R)-2-amino-3,3-dimethyl-butyric acid amide

-
-
20859-02-3
L-tert-Leucine

| Conditions | Yield |
|---|---|
| 92% |

-
-
20859-02-3
L-tert-Leucine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium nitrite In water at 40℃; for 11.75h; | 90.6% |
-
-
62965-57-5
(S)-(+)-2-amino-3,3-dimethylbutanamide

-
-
20859-02-3
L-tert-Leucine

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 100℃; for 24h; | 86% |
| With hydrogenchloride at 100℃; | 76% |
-
-
815-17-8
trimethylpyruvic acid

-
-
20859-02-3
L-tert-Leucine

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; D-glucose; ammonium chloride; sodium hydroxide In water for 5.5h; pH=8.3; Time; Reagent/catalyst; Enzymatic reaction; stereoselective reaction; | 80% |
| With L-ornithine; L-glutamic acid; Bacillus subtilis ornithine aminotransferase; Escherichia coli branched-chain amino acid aminotransferase; sodium hydroxide In aq. phosphate buffer at 37℃; for 16h; pH=8.5; Reagent/catalyst; Concentration; Time; Enzymatic reaction; | 73% |
| With L-valine; leucine dehydrogenase; pyridoxal 5'-phosphate; alcohol dehydrogenases from Bacillus stearothermophilus; branched-chain amino acid aminotransferase; NADH In isopropyl alcohol at 35℃; for 9h; pH=8; Reagent/catalyst; Enzymatic reaction; enantioselective reaction; | 68.7% |

-
-
20859-02-3
L-tert-Leucine

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon In methanol at 50℃; for 12h; Inert atmosphere; | 80% |
-
-
145058-02-2
(S)-2-<(R)-2-hydroxy-1-phenylethylamino>-3,3-dimethylbutanoic acid

-
-
20859-02-3
L-tert-Leucine

| Conditions | Yield |
|---|---|
| With formic acid; palladium In methanol; water Ambient temperature; | 76% |
-
-
162377-68-6
(S)-2-benzoylamino-3,3-dimethylbutyric acid

-
-
20859-02-3
L-tert-Leucine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; Amberlite IRA-67 1.) propan-2-ol, 70 deg C, 15 h; 2.) water, 15 h; | 74% |
| With potassium hydroxide In methanol; water; acetone |
-
-
126402-95-7
L-tert-leucine tert-butylamide hydrochloride

-
-
20859-02-3
L-tert-Leucine

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 80℃; for 24h; | 70% |
-
-
528819-01-4
N-[(1R)-2,2-dimethyl-1-phenylpropyl]-2,2,2-trifluoroacetamide

-
-
20859-02-3
L-tert-Leucine

| Conditions | Yield |
|---|---|
| Stage #1: N-[(1R)-2,2-dimethyl-1-phenylpropyl]-2,2,2-trifluoroacetamide With ruthenium trichloride; sodium periodate In tetrachloromethane; water; acetonitrile at 20℃; for 24h; Sharpless oxidation; Stage #2: With sodium hydroxide In methanol at 20℃; for 16h; | 68% |
-
-
1340309-12-7
N-succinyl-DL-tert-leucine

-
-
20859-02-3
L-tert-Leucine

| Conditions | Yield |
|---|---|
| 45% | |
| CoCl2 |

-
-
20859-02-3
L-tert-Leucine

| Conditions | Yield |
|---|---|
| With sodium hydroxide; manganese(ll) chloride; leucine aminopeptidase In water at 37℃; for 40h; | 35% |
-
-
62965-57-5, 113582-42-6, 144177-62-8
(±)-2-amino-3,3-dimethylbutanamide

-
-
20859-02-3
L-tert-Leucine

| Conditions | Yield |
|---|---|
| mit Hilfe eines Enzym-Praeparats aus Schweine-Nieren; | |
| Multi-step reaction with 3 steps 1: chlorocarbonic acid benzyl ester 2: amidase-substance from pig's kidney 3: palladium; methanol; acetic acid / Hydrogenation View Scheme |
-
-
92571-61-4
L-2-formylamino-3,3-dimethyl-butyric acid

-
-
20859-02-3
L-tert-Leucine

| Conditions | Yield |
|---|---|
| With hydrogen bromide |
-
-
62965-10-0
(S)-N-carbobenzoxy-tert-butylleucine

-
-
20859-02-3
L-tert-Leucine

| Conditions | Yield |
|---|---|
| With methanol; palladium; acetic acid Hydrogenation; | |
| (hydrogenolysis); |
-
-
3850-31-5
methyl tert-leucinate

-
-
20859-02-3
L-tert-Leucine

| Conditions | Yield |
|---|---|
| (i) liq. NH3, (ii) (racemate resolution using D-tartaric acid), (iii) aq. HCl; Multistep reaction; |
-
-
63038-26-6
(S)-2-amino-3,3-dimethyl-butyric acid methyl ester

-
-
20859-02-3
L-tert-Leucine

| Conditions | Yield |
|---|---|
| With hydrogenchloride |
-
-
22146-59-4
(S)-2-acetylamino-3,3-dimethylbutyric acid

-
-
20859-02-3
L-tert-Leucine

| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethanol |

-
-
20859-02-3
L-tert-Leucine

| Conditions | Yield |
|---|---|
| With hydrogenchloride |
-
-
38559-30-7
3,3-dimethyl-2-oxo-butanoic acid phenyhydrazone

-
-
20859-02-3
L-tert-Leucine

| Conditions | Yield |
|---|---|
| hydrogenation; Yield given; |
-
-
111525-71-4
(S)-α-azido-3,3-dimethyl butanoic acid

-
-
20859-02-3
L-tert-Leucine

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In ethyl acetate under 760 Torr; for 13h; Yield given; |

-
-
20859-02-3
L-tert-Leucine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; Amberlite IR-120 1.) 80 deg C; Yield given. Multistep reaction; |
-
-
121703-98-8
chloroacetyl-D,L-tert-leucine

-
A
-
20859-02-3
L-tert-Leucine

-
B
-
121703-98-8
(R)-2-(2-Chloro-acetylamino)-3,3-dimethyl-butyric acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide; potassium phosphate buffer; porcine kidney acylase I at 40℃; relative initial rate of hydrolysis, also with Aspergillus acylase I as a catalyst; with or without CoCl2; |

-
A
-
20859-02-3
L-tert-Leucine

-
B
-
26782-71-8
D-tert-leucine

| Conditions | Yield |
|---|---|
| With hydrogenchloride Yield given. Yields of byproduct given. Title compound not separated from byproducts; |

-
A
-
20859-02-3
L-tert-Leucine

-
B
-
26782-71-8
D-tert-leucine

| Conditions | Yield |
|---|---|
| With ammonia; lithium In tetrahydrofuran; tert-butyl alcohol at -78℃; for 0.5h; Yield given. Title compound not separated from byproducts; |
-
-
20859-02-3
L-tert-Leucine

-
-
501-53-1
benzyl chloroformate

-
-
62965-10-0
(S)-N-carbobenzoxy-tert-butylleucine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In 1,4-dioxane at 0℃; for 18h; | 100% |
| With sodium hydroxide In water for 3h; Inert atmosphere; | 100% |
| With sodium hydroxide In water at 5 - 20℃; for 14h; pH=10 - 10.5; Product distribution / selectivity; | 97% |

| Conditions | Yield |
|---|---|
| With thionyl chloride at 0 - 20℃; | 100% |
| With thionyl chloride for 16h; Reflux; | 100% |
| With hydrogenchloride Ambient temperature; | 98% |
-
-
20859-02-3
L-tert-Leucine

-
-
112245-13-3
(S)-tert-leucinol

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; iodine In tetrahydrofuran for 24h; Heating; | 100% |
| Stage #1: L-tert-Leucine With iodine In tetrahydrofuran for 24h; Reflux; Stage #2: With methanol Stage #3: With potassium hydroxide In water | 100% |
| With sodium tetrahydroborate; iodine In tetrahydrofuran at 0 - 80℃; for 22h; Inert atmosphere; | 100% |
-
-
20859-02-3
L-tert-Leucine

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
62965-35-9
N-tert-butyloxycarbonyl-L-tert-leucine

| Conditions | Yield |
|---|---|
| With triethylamine In methanol at 0 - 5℃; | 100% |
| With triethylamine In methanol at 0 - 5℃; | 100% |
| With sodium hydroxide In 1,4-dioxane at 20℃; for 12h; | 100% |
-
-
20859-02-3
L-tert-Leucine

-
-
79-22-1
methyl chloroformate

-
-
162537-11-3
(S)-2-(methoxycarbonylamino)-3,3-dimethylbutanoic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In 1,4-dioxane; water at 60℃; for 18h; pH=8 - 9; | 100% |
| With sodium hydroxide In 1,4-dioxane; water at 25 - 60℃; for 22h; | 98% |
| Stage #1: L-tert-Leucine; methyl chloroformate With sodium hydroxide In 1,4-dioxane; water at 25 - 60℃; for 22h; Stage #2: With hydrogenchloride In water | 98% |
-
-
13139-17-8
N-(Benzyloxycarbonyloxy)succinimide

-
-
20859-02-3
L-tert-Leucine

-
-
62965-10-0
(S)-N-carbobenzoxy-tert-butylleucine

| Conditions | Yield |
|---|---|
| With triethylamine In methanol at 20℃; for 14h; | 100% |
-
-
20859-02-3
L-tert-Leucine

-
-
501-52-0
3-Phenylpropionic acid

-
-
205494-44-6
N-(3-phenylpropanoyl)-L-tert-Leu-OH

| Conditions | Yield |
|---|---|
| Stage #1: 3-Phenylpropionic acid With chloroformic acid ethyl ester; triethylamine In tetrahydrofuran at 0℃; for 0.5h; Green chemistry; Stage #2: L-tert-Leucine With water In tetrahydrofuran at 0℃; for 0.5h; Green chemistry; | 100% |

-
-
20859-02-3
L-tert-Leucine

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
62965-35-9
N-tert-butyloxycarbonyl-L-tert-leucine

| Conditions | Yield |
|---|---|
| With triethylamine In 1,4-dioxane; water | 99% |
| With triethylamine In 1,4-dioxane; water | 99% |

-
-
20859-02-3
L-tert-Leucine

| Conditions | Yield |
|---|---|
| With triethylamine In toluene for 1.25h; Reflux; | 99% |
-
-
20859-02-3
L-tert-Leucine

-
-
1985-37-1
4-phenylnaphtho[2,3-c]furan-1,3-dione

| Conditions | Yield |
|---|---|
| With triethylamine In toluene for 12h; Inert atmosphere; Reflux; | 99% |
| In N,N-dimethyl-formamide Inert atmosphere; Reflux; | 89% |
-
-
20859-02-3
L-tert-Leucine

-
-
72607-35-3
3-(tert-butyl)-5-nitrosalicylaldehyde

-
-
1686-22-2
vanadium(V) oxytriethoxide

| Conditions | Yield |
|---|---|
| In methanol; dichloromethane for 3h; Inert atmosphere; | 99% |

-
-
20859-02-3
L-tert-Leucine

-
-
72607-35-3
3-(tert-butyl)-5-nitrosalicylaldehyde

-
-
5588-84-1
vanadium(V) oxytriisopropoxide

| Conditions | Yield |
|---|---|
| In methanol; dichloromethane for 3h; Inert atmosphere; | 99% |
| Stage #1: L-tert-Leucine; 3-(tert-butyl)-5-nitrosalicylaldehyde In methanol; dichloromethane for 3h; Reflux; Inert atmosphere; Stage #2: vanadium(V) oxytriisopropoxide In dichloromethane for 3h; Inert atmosphere; | 99% |

-
-
20859-02-3
L-tert-Leucine

-
-
72607-35-3
3-(tert-butyl)-5-nitrosalicylaldehyde

-
-
7681-91-6
oxovanadium(V) trimethoxide

| Conditions | Yield |
|---|---|
| Stage #1: L-tert-Leucine; 3-(tert-butyl)-5-nitrosalicylaldehyde In methanol; dichloromethane Reflux; Inert atmosphere; Stage #2: oxovanadium(V) trimethoxide In methanol; dichloromethane for 3h; Inert atmosphere; | 99% |


| Conditions | Yield |
|---|---|
| With coronafacic acid ligase SsCfaL for 20h; Catalytic behavior; Reagent/catalyst; Enzymatic reaction; | 99% |
-
-
20859-02-3
L-tert-Leucine

-
-
1609-86-5
Tert-butyl isocyanate

-
-
101968-85-8
(S)-2-(3-(tert-butyl)ureido)-3,3-dimethylbutanoic acid

| Conditions | Yield |
|---|---|
| Stage #1: L-tert-Leucine With sodium hydroxide In water pH=11.5; Stage #2: Tert-butyl isocyanate In water pH=9.3; Cooling with ice; | 98% |
-
-
20859-02-3
L-tert-Leucine

-
-
24327-08-0
meso-endo-tetrahydro-4,7-ethanoisobenzofuran-1,3-dione

| Conditions | Yield |
|---|---|
| With triethylamine In toluene for 12h; Inert atmosphere; Reflux; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: L-tert-Leucine; salicylaldehyde In water at 90℃; for 1h; Stage #2: phenylboronic acid In water at 90℃; for 20h; | 97% |

-
-
20859-02-3
L-tert-Leucine

-
-
28920-43-6
(fluorenylmethoxy)carbonyl chloride

-
-
132684-60-7
(S)-2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-3,3-dimethylbutanoic acid

| Conditions | Yield |
|---|---|
| With sodium carbonate In 1,4-dioxane at 0 - 20℃; | 96% |
| With sodium carbonate In 1,4-dioxane; water at 0 - 20℃; Inert atmosphere; | |
| With sodium carbonate In 1,4-dioxane; water at 0 - 20℃; for 1h; Inert atmosphere; | |
| With sodium carbonate In 1,4-dioxane at 0℃; for 2h; |
-
-
32703-79-0
4-tert-butylphthalic anhydride

-
-
20859-02-3
L-tert-Leucine

| Conditions | Yield |
|---|---|
| With triethylamine In toluene for 12h; Inert atmosphere; Reflux; | 96% |
| With triethylamine In toluene for 12h; Inert atmosphere; Reflux; | 96% |
-
-
20859-02-3
L-tert-Leucine

-
-
72607-35-3
3-(tert-butyl)-5-nitrosalicylaldehyde

-
-
1686-22-2
vanadium(V) oxytriethoxide

| Conditions | Yield |
|---|---|
| Stage #1: L-tert-Leucine; 3-(tert-butyl)-5-nitrosalicylaldehyde In ethanol; dichloromethane Inert atmosphere; Reflux; Stage #2: vanadium(V) oxytriethoxide In ethanol; dichloromethane at 20℃; for 3h; Inert atmosphere; | 96% |

-
-
20859-02-3
L-tert-Leucine

-
-
82911-69-1
N-(9H-fluoren-2-ylmethoxycarbonyloxy)succinimide

-
-
132684-60-7
(S)-2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-3,3-dimethylbutanoic acid

| Conditions | Yield |
|---|---|
| With sodium carbonate In water; acetone | 95% |

| Conditions | Yield |
|---|---|
| In methanol for 24h; | 95% |

-
-
20859-02-3
L-tert-Leucine

-
-
497-19-8
sodium carbonate

-
-
41715-33-7
3,5-di-tert-amyl-2-hydroxy-benzaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: L-tert-Leucine; sodium carbonate; 3,5-di-tert-amyl-2-hydroxy-benzaldehyde In ethanol at 90℃; for 6h; Stage #2: cobalt(II) nitrate hexahydrate In ethanol at 90℃; for 42h; | 95% |

-
-
85-44-9
phthalic anhydride

-
-
20859-02-3
L-tert-Leucine

-
-
142765-23-9
(S)-(-)-2-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-3,3-dimethyl-butyric acid

| Conditions | Yield |
|---|---|
| With triethylamine In toluene for 2h; Heating; | 94% |
| With triethylamine In toluene for 0.5h; Heating; | 86% |
| With triethylamine In toluene at 130℃; Dean-Stark; | 67% |
-
-
20859-02-3
L-tert-Leucine

-
-
50715-28-1
cyclopentyl chloroformate

-
-
572924-00-6
N-[(cyclopentyloxy)carbonyl]-L-tert-leucine

| Conditions | Yield |
|---|---|
| Stage #1: L-tert-Leucine With trimethylsilyl cyanide In acetonitrile for 0.25h; Stage #2: cyclopentyl chloroformate In acetonitrile at 80℃; | 94% |
| Stage #1: L-tert-Leucine With trimethylsilyl cyanide In acetonitrile at 75℃; for 0.75h; Stage #2: cyclopentyl chloroformate In acetonitrile at 80℃; | 94% |
L-tert-Leucine Chemical Properties
Molecule structure of L-tert-Leucine (CAS NO.20859-02-3):
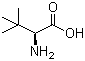
IUPAC Name: 2-Amino-3,3-dimethylbutanoic acid
Molecular Weight: 131.17292 g/mol
Molecular Formula: C6H13NO2
Density: 1.038 g/cm3
Melting Point: 300 °C
Boiling Point: 217.7 °C at 760 mmHg
Flash Point: 85.5 °C
Index of Refraction: 1.464
Molar Refractivity: 34.87 cm3
Molar Volume: 126.3 cm3
Surface Tension: 38.2 dyne/cm
Enthalpy of Vaporization: 50.03 kJ/mol
Vapour Pressure: 0.0499 mmHg at 25 °C
Storage Temp.: store at RT.
Water Solubility: 125.5 g/L (20 °C)
XLogP3: -1.8
H-Bond Donor: 2
H-Bond Acceptor: 3
Rotatable Bond Count: 2
Exact Mass: 131.094629
MonoIsotopic Mass: 131.094629
Topological Polar Surface Area: 63.3
Heavy Atom Count: 9
Canonical SMILES: CC(C)(C)C(C(=O)O)N
InChI: InChI=1S/C6H13NO2/c1-6(2,3)4(7)5(8)9/h4H,7H2,1-3H3,(H,8,9)
InChIKey: NPDBDJFLKKQMCM-UHFFFAOYSA-N
EINECS: 200-522-0
Product Categories: Amino acids, non natural; Chiral Compounds; API intermediates; PROTECTED AMINO ACID & PEPTIDES; Amino Acids; Leucine [Leu, L]; API; Peptide
L-tert-Leucine Uses
L-tert-Leucine (CAS NO.20859-02-3) is used as nutrient supplement and animal feed additives. It is also used for drug synthetic.
L-tert-Leucine Safety Profile
Hazard Codes:  Xi
Xi
Safety Statements: 22-24/25
S22:Do not breathe dust.
S24/25:Avoid contact with skin and eyes.
WGK Germany: 3
RTECS: OH2850000
Hazard Note: Irritant
L-tert-Leucine Specification
L-tert-Leucine (CAS NO.20859-02-3) is also named as (2S)-2-Amino-3,3-Dimethylbutanoic Acid ; (S)-2-Amino-3,3-dimethylbutanoic acid ; (S)-2-Amino-4-methylpentanoic acid ; 3-Methyl-L-valin ; 3-Methyl-L-valine . L-tert-Leucine (CAS NO.20859-02-3) is white to almost white powder.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View