-
Name
Methyl isobutyrylacetate
- EINECS 418-900-0
- CAS No. 42558-54-3
- Article Data26
- CAS DataBase
- Density 0.993 g/cm3
- Solubility 43.6g/L at 20℃
- Melting Point -75 °C
- Formula C7H12O3
- Boiling Point 185.8 °C at 760 mmHg
- Molecular Weight 144.17
- Flash Point 68.9 °C
- Transport Information
- Appearance colorless to yellowish liquid
- Safety 26-36
- Risk Codes 36/38
-
Molecular Structure
- Hazard Symbols 36/38:;
- Synonyms Methyl 4-methyl-3-oxovalerate;IBEM;
- PSA 43.37000
- LogP 0.77460
Synthetic route
-
-
563-80-4
3-methyl-butan-2-one

-
-
616-38-6
carbonic acid dimethyl ester

-
A
-
16466-21-0
2,5,6-trimethylhept-4-en-3-one

-
B
-
101459-87-4
2,5,6-trimethylhept-5-en-3-one

-
C
-
42558-54-3
Methyl 4-methyl-3-oxopentanoate

| Conditions | Yield |
|---|---|
| Stage #1: 3-methyl-butan-2-one With sodium hydride In 1,4-dioxane; mineral oil at 20℃; for 0.166667h; Stage #2: carbonic acid dimethyl ester In 1,4-dioxane; mineral oil at 50℃; for 3h; Reagent/catalyst; Solvent; Temperature; Time; | A n/a B n/a C 88% |


| Conditions | Yield |
|---|---|
| yttria-stabilized zirconia In 1,2-dichloro-ethane for 10h; Heating; | 85% |
| With Cu(II)-Mont K 10 clay In benzene at 80℃; for 3h; Condensation; | 62% |
-
-
563-80-4
3-methyl-butan-2-one

-
-
616-38-6
carbonic acid dimethyl ester

-
-
42558-54-3
Methyl 4-methyl-3-oxopentanoate

| Conditions | Yield |
|---|---|
| Stage #1: 3-methyl-butan-2-one With sodium hydride In tetrahydrofuran for 0.333333h; Stage #2: carbonic acid dimethyl ester In tetrahydrofuran at 30℃; Further stages.; | 85% |
| Stage #1: 3-methyl-butan-2-one; carbonic acid dimethyl ester With sodium hydride In toluene at 80℃; for 5h; Stage #2: With water; acetic acid In toluene | 62.72% |
| Stage #1: carbonic acid dimethyl ester With sodium hydride In toluene Reflux; Inert atmosphere; Stage #2: 3-methyl-butan-2-one In toluene Reflux; Inert atmosphere; |
-
-
67-56-1
methanol

-
-
2033-24-1
cycl-isopropylidene malonate

-
-
79-30-1
isobutyryl chloride

-
-
42558-54-3
Methyl 4-methyl-3-oxopentanoate

| Conditions | Yield |
|---|---|
| Stage #1: cycl-isopropylidene malonate; isobutyryl chloride With pyridine In dichloromethane at 20℃; for 2h; Stage #2: methanol In dichloromethane for 2h; Further stages.; | 75% |

-
-
50652-78-3
methyl 4-methyl-2-pentenoate

-
-
42558-54-3
Methyl 4-methyl-3-oxopentanoate

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; sodium tetrachloropalladate(II) In acetic acid; tert-butyl alcohol at 50℃; for 3h; | 64% |
| With methanol; water; oxygen; toluene-4-sulfonic acid; palladium dichloride In N,N-dimethyl acetamide at 80℃; under 4560.31 Torr; for 10h; Autoclave; regioselective reaction; | 68 %Chromat. |
-
-
563-80-4
3-methyl-butan-2-one

-
-
124-41-4
sodium methylate

-
-
105-58-8
Diethyl carbonate

-
A
-
42558-54-3
Methyl 4-methyl-3-oxopentanoate

-
B
-
7152-15-0
ethyl 4-methyl-3-oxo-pentanoate

-
-
186581-53-3, 908094-01-9
diazomethane

-
-
36577-33-0
(1R,3aS,8aS)-7-isopropyl-1,4-dimethyl-1,2,3,3a,6,8a-hexahydroazulene

-
-
42558-54-3
Methyl 4-methyl-3-oxopentanoate

| Conditions | Yield |
|---|---|
| (i) O3, AcOEt, (ii) aq. H2O2, KHCO3, (iii) /BRN= 102415/, Et2O; Multistep reaction; |
-
-
79322-87-5
ethyl 2-acetyl-4-methyl-3-oxopentanoate

-
-
124-41-4
sodium methylate

-
-
42558-54-3
Methyl 4-methyl-3-oxopentanoate

| Conditions | Yield |
|---|---|
| In methanol |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
5650-76-0
isobutyrylacetic acid

-
-
42558-54-3
Methyl 4-methyl-3-oxopentanoate

| Conditions | Yield |
|---|---|
| In diethyl ether Yield given; |
-
-
67-56-1
methanol

-
-
84794-38-7
5-(1-hydroxy-2-methylpropylidene)-2,2-dimethyl-1,3-dioxane-4,6-dione

-
-
42558-54-3
Methyl 4-methyl-3-oxopentanoate

| Conditions | Yield |
|---|---|
| for 4h; Heating; Yield given; |
-
-
67-56-1
methanol

-
-
74965-86-9
2,2-dimethyl-5-(2-methylpropanoyl)-1,3-dioxane-4,6-dione

-
-
42558-54-3
Methyl 4-methyl-3-oxopentanoate

| Conditions | Yield |
|---|---|
| for 4h; Heating; |
-
-
563-80-4
3-methyl-butan-2-one

-
-
42558-54-3
Methyl 4-methyl-3-oxopentanoate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: dimethylformamide / 6 h / 110 °C 2: diethyl ether View Scheme | |
| Multi-step reaction with 2 steps 1: dimethylformamide / 3 h / 110 °C 2: diethyl ether View Scheme |
-
-
42558-54-3
Methyl 4-methyl-3-oxopentanoate

| Conditions | Yield |
|---|---|
| With cat; hydrogen In methanol; dichloromethane at 25℃; under 77572.2 Torr; for 48h; | 100% |
| With hydrogen In methanol at 50℃; under 31029.7 Torr; for 24h; optical yield given as %ee; enantioselective reaction; | 99% |
| With C50H46Cl2N2O2P2Ru; hydrogen In methanol at 50℃; under 30003 Torr; for 24h; Catalytic behavior; Reagent/catalyst; Autoclave; enantioselective reaction; | 97.8% |
-
-
42558-54-3
Methyl 4-methyl-3-oxopentanoate

-
-
459-57-4
4-fluorobenzaldehyde

| Conditions | Yield |
|---|---|
| With piperidine; acetic acid In hexane at 20℃; for 12 - 16h; Heating / reflux; | 100% |

| Conditions | Yield |
|---|---|
| With lithium hydroxide In water at 0℃; for 0.3h; | 100% |
-
-
42558-54-3
Methyl 4-methyl-3-oxopentanoate

-
-
619-45-4
4-methoxycarbonyl aniline

-
-
936847-59-5
methyl 4-(4-methyl-3-oxopentanamido)benzoate

| Conditions | Yield |
|---|---|
| With ethylenediamine In toluene for 20 - 25h; Heating / reflux; | 99.45% |
| In toluene for 24h; Reflux; Dean-Stark; | 85% |
-
-
42558-54-3
Methyl 4-methyl-3-oxopentanoate

-
-
76835-65-9
(R)-3-Hydroxy-3-methylpentansaeure-methylester

| Conditions | Yield |
|---|---|
| With hydrogen; (S)-BinapRuBr2 In methanol at 60℃; under 760.051 Torr; for 18h; Noyori reduction; | 99% |
| With RuBr2{(S)-2,2'-bis(diphenylphosphino)-1,1'-dinaphthyl}; hydrogen In methanol at 60℃; under 760.051 Torr; | 94% |
| With hydrogen In methanol at 80℃; under 15001.5 Torr; for 10h; enantioselective reaction; | 91.9% |
-
-
42558-54-3
Methyl 4-methyl-3-oxopentanoate

-
-
2158-14-7
4-acetamidobenzenesulfonyl azide

-
-
402568-04-1
methyl 2-diazo-4-methyl-3-oxopentanoate

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 20℃; for 16h; | 99% |

| Conditions | Yield |
|---|---|
| With sulfuric acid In methanol at 65℃; for 48h; Reagent/catalyst; | 98% |
| With sulfuric acid; copper(l) chloride In methanol at 78℃; for 9h; | 94.5% |
| With sulfuric acid; copper(l) chloride In methanol at 80℃; for 24h; Biginelli Pyrimidone Synthesis; Inert atmosphere; | 86% |

-
-
42558-54-3
Methyl 4-methyl-3-oxopentanoate

-
-
29068-23-3
3-isopropylisoxazol-5(4H)-one

| Conditions | Yield |
|---|---|
| Stage #1: Methyl 4-methyl-3-oxopentanoate With hydroxylamine hydrochloride; sodium acetate In ethanol Reflux; Stage #2: With hydrogenchloride In ethanol; water Reflux; | 98% |
| With hydroxylamine hydrochloride; sodium acetate In ethanol at 78℃; | 78% |

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol at 20℃; for 18h; | 98% |
-
-
5153-67-3
nitrostyrene

-
-
42558-54-3
Methyl 4-methyl-3-oxopentanoate

| Conditions | Yield |
|---|---|
| [(S,S)-N-(pentamethylbenzenesulfonyl)-1,2-diphenylethylenediamine] (hexamethylbenzene)ruthenium at -20℃; for 48h; Michael Condensation; | 97% |
-
-
42558-54-3
Methyl 4-methyl-3-oxopentanoate

-
-
4637-24-5
N,N-dimethyl-formamide dimethyl acetal

| Conditions | Yield |
|---|---|
| at 80℃; for 1h; | 97% |
-
-
42558-54-3
Methyl 4-methyl-3-oxopentanoate

-
-
3599-89-1
propionamidine hydrochloride

-
-
34127-00-9
2‐ethyl‐6‐isopropylpyrimidin‐4(3H)‐one

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol at 20℃; Inert atmosphere; | 97% |

| Conditions | Yield |
|---|---|
| With [Ir(cod)2]SbF6; 2,2'-bis(diphenylphosphino)biphenyl for 24h; Catalytic behavior; Reagent/catalyst; Reflux; diastereoselective reaction; | 97% |
-
-
757251-39-1
4-(4-amino-3-fluorophenoxy)pyridine-2-carboxylic acid methyl amide

-
-
42558-54-3
Methyl 4-methyl-3-oxopentanoate

-
-
320-51-4
4-chloro-3-trifluoromethyl-aniline

-
-
755037-03-7
regorafenib

| Conditions | Yield |
|---|---|
| With dmap In N,N-dimethyl-formamide at 140℃; for 4h; Temperature; Green chemistry; | 96.8% |

-
-
42558-54-3
Methyl 4-methyl-3-oxopentanoate

-
-
62-53-3
aniline

-
-
124401-38-3
4-methyl-3-oxo-N-phenylpentanamide

| Conditions | Yield |
|---|---|
| With dmap at 100℃; for 16h; | 96.4% |
| With triethylamine at 80 - 125℃; | 92.7% |
| With sodium hydroxide In neat (no solvent) at 135℃; for 12h; | 92% |
-
-
42558-54-3
Methyl 4-methyl-3-oxopentanoate

-
-
17356-08-0
thiourea

-
-
1504-75-2
3-(4-methylphenyl)acrylaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: thiourea; 4-methyl cinnamaldehyde With 1-(3,5-bis(trifluoromethyl)phenyl)-3-((S)-(6-methoxyquinolin-4-yl)((1S,2S,4S,5R)-5-vinylquinuclidin-2-yl)methyl)thiourea; (1R,3S,5R)-2-azabicyclo[3.1.0]hexane-3 -carboxylic acid In dichloromethane at 20℃; for 3h; Biginelli Pyrimidone Synthesis; Stage #2: Methyl 4-methyl-3-oxopentanoate In dichloromethane at 50℃; for 15h; Biginelli Pyrimidone Synthesis; enantioselective reaction; | 96% |


| Conditions | Yield |
|---|---|
| Stage #1: 4-methoxycinnamaldehyde; thiourea With quinidine thiourea A; (1R,3S,5R)-2-azabicyclo[3.1.0]hexane-3 -carboxylic acid In dichloromethane at 20℃; for 3h; Biginelli Pyrimidone Synthesis; Sealed tube; Stage #2: Methyl 4-methyl-3-oxopentanoate In dichloromethane at 50℃; for 15h; Biginelli Pyrimidone Synthesis; Sealed tube; enantioselective reaction; | 96% |
| Stage #1: 4-methoxycinnamaldehyde; thiourea With 1-(3,5-bis(trifluoromethyl)phenyl)-3-((S)-(6-methoxyquinolin-4-yl)((1S,2S,4S,5R)-5-vinylquinuclidin-2-yl)methyl)thiourea; (1R,3S,5R)-2-azabicyclo[3.1.0]hexane-3 -carboxylic acid In dichloromethane at 20℃; for 3h; Biginelli Pyrimidone Synthesis; Stage #2: Methyl 4-methyl-3-oxopentanoate In dichloromethane at 50℃; for 15h; Biginelli Pyrimidone Synthesis; enantioselective reaction; | 96% |

-
-
42558-54-3
Methyl 4-methyl-3-oxopentanoate

-
-
459-57-4
4-fluorobenzaldehyde

-
-
122549-26-2
methyl 2-(4-fluorophenyl)methylidene-3-oxo-4-methylpentanoate

| Conditions | Yield |
|---|---|
| With piperidine; acetic acid In isopropyl alcohol at 20℃; for 22h; Inert atmosphere; | 95.4% |
| With piperidine; acetic acid In isopropyl alcohol at 25 - 35℃; for 20h; Knoevenagel Condensation; | 86.11% |
| With piperidine; acetic acid In isopropyl alcohol at 30℃; for 0.5h; Temperature; | 80% |
-
-
42558-54-3
Methyl 4-methyl-3-oxopentanoate

-
-
100-46-9
benzylamine

-
-
1225057-22-6
methyl 5-isopropyl-2-phenyloxazole-4-carboxylate

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; iodine In N,N-dimethyl-formamide at 80℃; for 6h; | 95% |
| With tert.-butylhydroperoxide; iodine; copper(II) acetate monohydrate In N,N-dimethyl-formamide at 20℃; | 91% |
| With tert-butyl hydroxyperoxide; tetra-(n-butyl)ammonium iodide In water; ethyl acetate at 40℃; for 8h; | 69% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-nitrobenzaldehdye; thiourea With 1-(3,5-bis(trifluoromethyl)phenyl)-3-((S)-(6-methoxyquinolin-4-yl)((2S,4S,8R)-8-vinylquinuclidin-2-yl)methyl)thiourea; (1R,3S,5R)-2-azabicyclo[3.1.0]hexane-3 -carboxylic acid In toluene at 20℃; for 3h; Biginelli Pyrimidone Synthesis; Stage #2: Methyl 4-methyl-3-oxopentanoate In toluene at 50℃; for 15h; Biginelli Pyrimidone Synthesis; enantioselective reaction; | 95% |

-
-
4743-17-3
5-Chloroisatoic anhydride

-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

-
-
42558-54-3
Methyl 4-methyl-3-oxopentanoate

| Conditions | Yield |
|---|---|
| Stage #1: 5-Chloroisatoic anhydride; Methyl 4-methyl-3-oxopentanoate With sodium hydride In N,N-dimethyl acetamide at 0 - 120℃; for 1h; Stage #2: N,N-phenylbistrifluoromethane-sulfonimide With sodium hydride In N,N-dimethyl-formamide at 20℃; for 2h; | 95% |


| Conditions | Yield |
|---|---|
| With ZnCl2 supported on Fe3O4 (at) SiO2 nanocatalyst In neat (no solvent) at 60℃; for 4h; | 95% |
-
-
42558-54-3
Methyl 4-methyl-3-oxopentanoate

-
-
3800-06-4
2-amino-4'-fluorobenzophenone

-
-
130954-89-1
methyl 4-(4-fluorophenyl)-2-(1-methylethyl)-3-quinolinecarboxylate

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid for 0.00833333h; Friedlaender condensation; microwave irradiation; | 94% |
| With toluene-4-sulfonic acid In toluene for 5h; Heating; | 28% |
-
-
42558-54-3
Methyl 4-methyl-3-oxopentanoate

-
-
771477-49-7
(E)-3-benbenzylidene-5-phenylpent-4-yn-2-one

-
-
1175095-87-0
C25H26O4

| Conditions | Yield |
|---|---|
| With [Pd(dppp)(H2O)2](2+)*2(OTf)(1-) In 1,2-dichloro-ethane at 20℃; for 10h; tandem Michael addition-cyclization; optical yield given as %de; diastereoselective reaction; | 94% |
-
-
455-19-6
4-Trifluoromethylbenzaldehyde

-
-
42558-54-3
Methyl 4-methyl-3-oxopentanoate

-
-
17356-08-0
thiourea

| Conditions | Yield |
|---|---|
| Stage #1: 4-Trifluoromethylbenzaldehyde; thiourea With 1-(3,5-bis(trifluoromethyl)phenyl)-3-((S)-(6-methoxyquinolin-4-yl)((2S,4S,8R)-8-vinylquinuclidin-2-yl)methyl)thiourea; (1R,3S,5R)-2-azabicyclo[3.1.0]hexane-3 -carboxylic acid In toluene at 20℃; for 3h; Biginelli Pyrimidone Synthesis; Stage #2: Methyl 4-methyl-3-oxopentanoate In toluene at 50℃; for 15h; Biginelli Pyrimidone Synthesis; enantioselective reaction; | 94% |

-
-
42558-54-3
Methyl 4-methyl-3-oxopentanoate

-
-
459-57-4
4-fluorobenzaldehyde

-
-
109-77-3
malononitrile

-
-
954153-13-0
C17H17FN2O3

| Conditions | Yield |
|---|---|
| With piperidine In ethanol at 20 - 76℃; for 1h; | 93% |


-
-
42558-54-3
Methyl 4-methyl-3-oxopentanoate

| Conditions | Yield |
|---|---|
| With copper(l) iodide; caesium carbonate; L-proline In dimethyl sulfoxide at 60℃; for 12h; | 93% |

| Conditions | Yield |
|---|---|
| With silver carbonate In 1,4-dioxane at 80℃; | 93% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-chlorobenzaldehyde; thiourea With 1-(3,5-bis(trifluoromethyl)phenyl)-3-((S)-(6-methoxyquinolin-4-yl)((2S,4S,8R)-8-vinylquinuclidin-2-yl)methyl)thiourea; (1R,3S,5R)-2-azabicyclo[3.1.0]hexane-3 -carboxylic acid In toluene at 20℃; for 3h; Biginelli Pyrimidone Synthesis; Stage #2: Methyl 4-methyl-3-oxopentanoate In toluene at 50℃; for 15h; Biginelli Pyrimidone Synthesis; enantioselective reaction; | 93% |


| Conditions | Yield |
|---|---|
| Stage #1: 4-hydroxy-benzaldehyde; thiourea With 1-(3,5-bis(trifluoromethyl)phenyl)-3-((S)-(6-methoxyquinolin-4-yl)((2S,4S,8R)-8-vinylquinuclidin-2-yl)methyl)thiourea; (1R,3S,5R)-2-azabicyclo[3.1.0]hexane-3 -carboxylic acid In toluene at 20℃; for 3h; Biginelli Pyrimidone Synthesis; Stage #2: Methyl 4-methyl-3-oxopentanoate In toluene at 50℃; for 15h; Biginelli Pyrimidone Synthesis; enantioselective reaction; | 93% |

Methyl isobutyrylacetate Chemical Properties
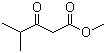
Molecular structure of Methyl isobutyrylacetate (CAS NO.42558-54-3) is:
Product Name: Methyl isobutyrylacetate
CAS Registry Number: 42558-54-3
Molecular Formula: C7H12O3
Molecular Weight: 144.17
EINECS: 418-900-0
Melting Point: -75°C
Surface Tension: 29.4 dyne/cm
Density: 0.993 g/cm3
Flash Point: 68.9 °C
Enthalpy of Vaporization: 42.2 kJ/mol
Boiling Point: 185.8 °C at 760 mmHg
Vapour Pressure: 0.686 mmHg at 25°C
Refractive index: 1.4265
Methyl isobutyrylacetate Uses
Methyl isobutyrylacetate (CAS NO.42558-54-3) can be used for the synthesis if heterocycles (Furane, Pyrazolone, Chinilone) and building block for pharmaceuticals (reducer of cholesterol).
Methyl isobutyrylacetate Safety Profile
Safty information about Methyl isobutyrylacetate (CAS NO.42558-54-3) is:
Risk Statements: 36/38
R36/38:Irritating to eyes and skin.
Safety Statements: 26-36
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
WGK Germany: 1
Methyl isobutyrylacetate Specification
Methyl isobutyrylacetate , its cas register number is 42558-54-3. It also can be called Methyl 4-methyl-3-oxovalerate ; IBEM .It is a colorless to yellowish liquid.
Related Products
- Methyl (((methoxymethylphosphinothioyl)thio)acetyl)methylcarbamate
- Methyl (+)-(3R)-7-[4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methanesulfonylamino)pyrimidin-5-yl]-3-hydroxy-5-oxo-(6E)-heptenoate
- Methyl (2R)-2-[(tert-butoxycarbonyl)amino]-3-iodopropanoate
- Methyl (2R)-2-[4-(2,4-dichlorophenoxy)phenoxy]propanoate
- Methyl (2R)-2-amino-2-cyclohexylethanoate hydrochloride
- Methyl (2R)-2-bromo-2-(2-chlorophenyl)acetate
- Methyl (2R,3S)-3-(4-methoxyphenyl)-2-oxiranecarboxylate
- Methyl (2R,3S)-3-(benzoylamino)-2-hydroxy-3-phenylpropanoate
- Methyl (2R,3S)-3-(tert-butoxycarbonylamino)-2-hydroxy-3-phenylpropionate
- Methyl (2S)-1-(1,2-dioxo-3,3-dimethypentyl)-2-pyrrolidinecarboxylate
- 425615-42-5
- 425-61-6
- 42561-71-7
- 425637-18-9
- 42564-41-0
- 42564-51-2
- 425663-71-4
- 425671-29-0
- 425680-98-4
- 425702-28-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View