-
Name
N,N,N',N'-Tetramethylethylenediamine
- EINECS 203-744-6
- CAS No. 110-18-9
- Article Data83
- CAS DataBase
- Density 0.817 g/cm3
- Solubility miscible with water
- Melting Point -55 °C(lit.)
- Formula C6H16N2
- Boiling Point 121 °C at 760 mmHg
- Molecular Weight 116.206
- Flash Point 10 °C
- Transport Information UN 2372 3/PG 2
- Appearance Colorless to slightly yellow liquid
- Safety 16-26-36/37/39-45
- Risk Codes 11-20/22-34-20/21/22
-
Molecular Structure
-
Hazard Symbols
 F,
F, C
C
- Synonyms 1,2-Ethanediamine,N,N,N',N'-tetramethyl- (9CI);Ethylenediamine, N,N,N',N'-tetramethyl-(6CI,8CI);(2-(Dimethylamino)ethyl)dimethylamine;1,2-Bis(dimethylamino)ethane;2,5-Dimethyl-2,5-diazahexane;Dabco TMEDA;Dimethyl[2-(dimethylamino)ethyl]amine;Kaolizer 11;MR;MR (amine);N,N,N',N'-Tetramethyl-1,2-diaminoethane;N,N,N',N'-Tetramethyl-1,2-ethanediamine;N,N,N',N'-Tetramethyl-1,2-ethylenediamine;Propamine D;TEMED;TMED;TMEDA;Tetrameen;Toyocat TE;
- PSA 6.48000
- LogP 0.10960
Synthetic route

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0 - 20℃; for 24h; | 100% |
| In chloroform at 20℃; for 24h; | 100% |

| Conditions | Yield |
|---|---|
| With 1-ethyl-2-pyrrolidinone; sodium stannate(IV) trihydrate; alumina; calcium hydroxide at 45 - 80℃; for 6h; Reagent/catalyst; Temperature; Autoclave; Inert atmosphere; | 98.6% |

| Conditions | Yield |
|---|---|
| With oxalic acid at 100 - 120℃; Eschweiler-Clarke methylation; | 96% |
| With hydrogen In methanol at 130℃; under 15001.5 Torr; for 4.5h; Autoclave; | 89% |
| With formic acid for 11h; Eschweiler-Clarke reaction; Reflux; | 80% |
| at 130 - 160℃; | |
| With water at 130 - 160℃; im Autoklaven; |

| Conditions | Yield |
|---|---|
| With hydrogen at 180℃; under 7500.75 Torr; | 91.6% |
| With hydrogen at 180℃; under 7500.75 Torr; | 91.6% |
-
-
89184-58-7
Li(1+)*((H3C)2NCH2)2*(C5H5)V(C3H5)2(1-)=(Li((H3C)2NCH2)2)((C5H5)V(C3H5)2)

-
-
201230-82-2
carbon monoxide

-
B
-
592-42-7
1,5-Hexadien

-
C
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran reaction of CO with (LiTMEDA)(CpV(C3H5)2) in THF (Ar); formation of 1,5-hexadiene in the reaction soln., extn. of the residue with toluene, filtering off the solid and drying to give Li2(CpV(CO)3), evapn. of the filtrate to dryness and subliming the residue in a high vacuum at 80°C to give CpV(CO)4; | A 91% B 46% C n/a D n/a |


| Conditions | Yield |
|---|---|
| In diethyl ether vigorously stirring a suspension of (3,4,5-η3)-3,4-dimethyl-3-pentenylato-N,N,N',N'-tetramethylethylenediaminenickel(II) in ether with 2,2'-bipyridine (CO2 or Ar); filtn. of the formed brown yellow ppt. after 24 h, shaking with THF fora few hours, filtn. and washing the residue with ether, drying in vacuo, elem. anal.; | A 90% B n/a |


-
A
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; for 0.333333h; Inert atmosphere; Schlenk technique; | A n/a B 86% |

-
-
141-46-8
Glycolaldehyde

-
-
124-40-3
dimethyl amine

-
A
-
108-01-0
2-(N,N-dimethylamino)ethanol

-
B
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

| Conditions | Yield |
|---|---|
| With platinum on carbon; hydrogen In ethanol at 25℃; under 30003 Torr; for 12h; Solvent; Pressure; Reagent/catalyst; Autoclave; | A 84% B 8% |
| With 5%-palladium/activated carbon; hydrogen In ethanol at 25 - 80℃; under 30003 Torr; for 3h; Autoclave; | A 35% B 40% |

-
-
1020084-83-6
[4-Et(C6H4)C(NSiMe3)2]Li(TMEDA)

-
-
10026-11-6
zirconium(IV) chloride

-
A
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

| Conditions | Yield |
|---|---|
| In toluene at 0℃; for 3h; Cooling; Schlenk technique; | A n/a B 83% C n/a |

-
-
1020084-88-1
[2-OMe(C6H4)C(NSiMe3)2]Li(TMEDA)

-
-
10026-11-6
zirconium(IV) chloride

-
A
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

| Conditions | Yield |
|---|---|
| In toluene at 0℃; for 3h; Cooling; Schlenk technique; | A n/a B 82% C n/a |


-
-
10026-11-6
zirconium(IV) chloride

-
A
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

| Conditions | Yield |
|---|---|
| In toluene at 0℃; for 3h; Cooling; Schlenk technique; | A n/a B 79% C n/a |

-
-
1020084-86-9
[4-OMe(C6H4)C(NSiMe3)2]Li(TMEDA)

-
-
10026-11-6
zirconium(IV) chloride

-
A
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

| Conditions | Yield |
|---|---|
| In toluene at 0℃; for 3h; Cooling; Schlenk technique; | A n/a B 78% C n/a |

-
-
1020084-84-7
[4-n-Bu(C6H4)C(NSiMe3)2]Li(TMEDA)

-
-
10026-11-6
zirconium(IV) chloride

-
A
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

| Conditions | Yield |
|---|---|
| In toluene at 0℃; for 3h; Cooling; Schlenk technique; | A n/a B 70% C n/a |

-
-
1206534-47-5
[(3-C4H3O)C(NSiMe3)2]Li(TMEDA)

-
-
10026-11-6
zirconium(IV) chloride

-
A
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

| Conditions | Yield |
|---|---|
| In toluene at 0℃; for 3h; Cooling; Schlenk technique; | A n/a B 68% C n/a |

-
-
112506-19-1
(tetramethylethylenediamine)lithium trihydro(2,4,6-tri-tert-butylphenyl)borate

-
A
-
112506-16-8
5,7-di-tert-butylhydroxy-3,3-dimethyl-1-boraindane

-
B
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water In diethyl ether; water byproducts: H2, LiCl; addn. of HCl to ether soln. of borate (0°C), stirring (room temp., 1 h), aq. HCl addn.; drying of etheral phase (Na2SO4), solvent removal (vacuum), repptn. (pentane); elem. anal.; | A 53% B n/a |
-
-
112506-19-1
(tetramethylethylenediamine)lithium trihydro(2,4,6-tri-tert-butylphenyl)borate

-
A
-
112506-15-7
hydroxy(2,4,6-tri-tert-butylphenyl)borane

-
B
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

| Conditions | Yield |
|---|---|
| With water In tert-butyl alcohol byproducts: H2, LiOH; stirring (0°C, 6h, room temp., 12 h); solvent removal (vacuum), addn. of ether/water, drying of ether phase (Na2SO4), solvent removal (vacuum), recrystn. (pentane); elem. anal.; | A 36% B n/a |

| Conditions | Yield |
|---|---|
| With methanol; nickel at 175 - 200℃; under 40452.9 Torr; Hydrogenation; |
-
-
50-00-0
formaldehyd

-
-
22029-36-3
ethylenediammonium sulfate

-
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

| Conditions | Yield |
|---|---|
| at 130 - 140℃; Destillation des Reaktionsprodukts mit Kalilauge; |
-
-
124-41-4
sodium methylate

-
-
19826-49-4
4,5-epoxy-3,6-dimethoxy-17,17-dimethyl-morphina-6,8-dienium; iodide

-
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

-
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

| Conditions | Yield |
|---|---|
| at 140℃; unter Druck; |
-
-
124-40-3
dimethyl amine

-
-
107-99-3
2-(dimethylamino)ethyl chloride

-
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

| Conditions | Yield |
|---|---|
| With xylene |
-
-
50-00-0
formaldehyd

-
-
64-18-6
formic acid

-
-
107-15-3
ethylenediamine

-
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

-
-
107-99-3
2-(dimethylamino)ethyl chloride

-
A
-
108-01-0
2-(N,N-dimethylamino)ethanol

-
B
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

| Conditions | Yield |
|---|---|
| With alkalies |

| Conditions | Yield |
|---|---|
| Multistep reaction; |
-
-
140-31-8
aminoethylpiperazine

-
-
50-00-0
formaldehyd

-
-
64-18-6
formic acid

-
-
107-06-2
1,2-dichloro-ethane

-
A
-
106-58-1
N,N'-dimethylpiperazine

-
B
-
280-57-9
1,4-diaza-bicyclo[2.2.2]octane

-
C
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

| Conditions | Yield |
|---|---|
| Mechanism; |

-
-
140-31-8
aminoethylpiperazine

-
-
50-00-0
formaldehyd

-
-
64-18-6
formic acid

-
-
107-06-2
1,2-dichloro-ethane

-
A
-
280-57-9
1,4-diaza-bicyclo[2.2.2]octane

-
B
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

| Conditions | Yield |
|---|---|
| Multistep reaction. Further byproducts given; |

-
-
41203-22-9
1,4,8,11-tetramethyl-1,4,8,11-tetra-azacyclotetradecane

-
A
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

| Conditions | Yield |
|---|---|
| In toluene at 25℃; Rate constant; |

-
-
32317-47-8
(1-ethyl-propenyl)-dimethyl-amine

-
-
71889-99-1
N,N,N',N'-tetramethyl-1,2-diaminoethane-H+

-
A
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

| Conditions | Yield |
|---|---|
| ICR double resonance, proton affinity; |

-
-
20916-47-6
N,N-Dimethylisobutenylamin

-
-
71889-99-1
N,N,N',N'-tetramethyl-1,2-diaminoethane-H+

-
A
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

| Conditions | Yield |
|---|---|
| ICR double resonance, proton affinity; |

-
-
107-21-1
ethylene glycol

-
-
124-40-3
dimethyl amine

-
A
-
108-01-0
2-(N,N-dimethylamino)ethanol

-
B
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

| Conditions | Yield |
|---|---|
| With tris(triphenylphosphine)ruthenium(II) chloride at 120℃; for 3h; Yield given. Yields of byproduct given. Title compound not separated from byproducts; | |
| With ruthenium trichloride at 120℃; for 2.5h; Yield given. Yields of byproduct given. Title compound not separated from byproducts; |


| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 12h; | 100% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; pentane for 2h; Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: tetrahydrofuran; N,N,N,N,-tetramethylethylenediamine; diphenylphosphane With n-butyllithium In tetrahydrofuran; hexane Stage #2: With tellurium In tetrahydrofuran; hexane at -78 - 20℃; | 100% |


| Conditions | Yield |
|---|---|
| In acetonitrile; benzene Electrolysis; absence of oxygen and moisture; W-cathode, Zn suspended on Pt-anode, 30 V, 25 mA, 3.0 h, Et4NClO4 electrolyte; sepn. of pptd. product (further crop on addn. of petroleum ether), washing (petroleum ether), drying (vac.); elem. anal.; | 100% |


-
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

-
-
80533-23-9
[η**3-2-propenyl](N,N,N',N'-tetramethylethylenediamine)palladium(II) dichloro(η**3-2-propenyl)palladate(II)

| Conditions | Yield |
|---|---|
| In acetone TMEDA added to -50°C soln.; stirred 1 h at -50°C; solvent vac. removed; recrystd. from acetone/toluene at -20°C; elem. anal.; | 100% |
-
-
12146-37-1, 124717-04-0
(bicyclo[2.2.1]hepta-2,5-diene)tetracarbonylmolybdenum(0)

-
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

-
A
-
23301-98-6
(N,N,N',N'-tetramethylethylenediamine)tetracarbonylmolybdenum(0)

-
B
-
121-46-0
bicyclo[2.2.1]hepta-2,5-diene

| Conditions | Yield |
|---|---|
| In tetrahydrofuran reaction in a calorimeter under argon; | A 100% B n/a |


-
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

-
-
127161-53-9
LiOB(CH(SiMe3)2)2((Me2NCH2)2)

| Conditions | Yield |
|---|---|
| In hexane react. at 25°C; filtering, filtrate at -20°C; | 100% |
-
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

-
-
7550-45-0
titanium tetrachloride

-
-
19033-22-8
tetrachloro(N,N,N',N'-tetramethylethane-1,2-diamine)titanium(IV)

| Conditions | Yield |
|---|---|
| In hexane at 0℃; for 1h; Inert atmosphere; | 100% |
| In benzene solns. mixed at 10-20°C; filtered, washed (benzene), dried (vac.); elem. anal.; | >99 |
| In pentane solns. mixed at 10-20°C; filtered, washed (pentane), dried (vac.); elem. anal.; | >99 |

-
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

-
-
72049-53-7
N,N,N',N',-tetramethylethylenediamine*2AlH3

| Conditions | Yield |
|---|---|
| In diethyl ether at 25°C; ppt. collected by centrifugation, washed with Et2O, dried, elem. anal.; | 100% |

-
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

-
-
260066-12-4
[(η5-C5H5)RuCl(N,N,N',N'-tetramethylethylenediamine)]

| Conditions | Yield |
|---|---|
| In acetone soln. of Ru-complex and ligand in acetone was stirred at room temp. for 2 h under Ar; solvent was removed under vac., washed with pentane; | 100% |
-
-
636-98-6
p-nitrobenzene iodide

-
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

-
-
32005-36-0
bis(dibenzylideneacetone)-palladium(0)

-
-
191667-18-2
PdI(4-NO2C6H4)(N,N,N',N'-tetramethylethylenediamine)

| Conditions | Yield |
|---|---|
| In not given byproducts: dibenzylideneacetone; N2-atmosphere; according to Markies, A. J. et al.: J. Organomet. Chem. 482 (1994) 191; elem. anal.; | 100% |

-
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

-
-
13329-40-3
4-Iodoacetophenone

-
-
32005-36-0
bis(dibenzylideneacetone)-palladium(0)

-
-
191667-17-1
PdI(4-MeCOC6H4)(N,N,N',N'-tetramethylethylenediamine)

| Conditions | Yield |
|---|---|
| In not given byproducts: dibenzylideneacetone; N2-atmosphere; according to Markies, A. J. et al.: J. Organomet. Chem. 482 (1994) 191; elem. anal.; | 100% |

-
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

| Conditions | Yield |
|---|---|
| In acetone TMEDA added dropwise at -50°C, stirred 1 h at -50°C; solvent vac. evapd.; trituration with ether, dried in vac.; elem. anal.; | 100% |
-
-
14104-20-2
silver tetrafluoroborate

-
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran byproducts: AgCl; π-2-butenylpalladium chloride dimer dissolved in THF; AgBF4 added; stirred 10 min; AgCl removed and washed with THF; TMEDA added dropwise toTHF extracts, stirred 10 min; solvent vac. removed; elem. anal.; | 100% |

-
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

| Conditions | Yield |
|---|---|
| In acetone TMEDA added dropwise to -50°C soln.; stirred 1 h at -50°C; solvent vac. removed; elem. anal.; | 100% |
-
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

-
-
176650-93-4
3,5-bis(3,5-dimethoxybenzocarboxymethyl)benzyl bromide

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 70℃; Inert atmosphere; | 100% |
| In N,N-dimethyl-formamide at 70℃; for 15h; Inert atmosphere; | 100% |
-
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran excess N,N-tetramethylethylenediamine; | 100% |
-
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

-
-
639084-65-4
[ThCl4(1,2-dimethoxyethane)2]

-
-
159472-32-9
ThCl4(Me2NCH2CH2NMe2)2

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 0.5h; | 100% |
-
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

| Conditions | Yield |
|---|---|
| In toluene at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| at -78℃; for 0.333333h; Schlenk technique; Inert atmosphere; Reflux; | 100% |
| at -78℃; for 0.333333h; Inert atmosphere; Reflux; | 100% |

| Conditions | Yield |
|---|---|
| In chloroform at 85℃; for 24h; | 100% |

| Conditions | Yield |
|---|---|
| In chloroform at 85℃; for 24h; | 100% |

| Conditions | Yield |
|---|---|
| In chloroform at 85℃; for 24h; | 100% |
| In acetonitrile for 1h; Reflux; | 44% |
-
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

| Conditions | Yield |
|---|---|
| In hexane at 25℃; Inert atmosphere; Glovebox; | 100% |

| Conditions | Yield |
|---|---|
| In diethyl ether for 1h; | 100% |
-
-
18908-66-2
2-ethylhexyl bromide

-
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

| Conditions | Yield |
|---|---|
| for 12h; Reflux; | 100% |

-
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

| Conditions | Yield |
|---|---|
| Stage #1: N,N,N,N,-tetramethylethylenediamine; 2H2NO3(1-)*(x)H2O*Pd(2+) In dimethyl sulfoxide at 80℃; for 0.166667h; Stage #2: 1,3,5-tris(1-imidazolyl)benzene In dimethyl sulfoxide at 80℃; for 2h; | 100% |

-
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

-
-
32752-54-8
4-(bromomethyl)benzophenone

| Conditions | Yield |
|---|---|
| In chloroform at 20℃; for 72h; | 99.7% |
| In chloroform at 20℃; for 72h; | 99.7% |
| In chloroform |

-
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

-
-
22560-16-3
lithium triethylborohydride

| Conditions | Yield |
|---|---|
| In tetrahydrofuran byproducts: B(C2H5)3, H2; addn. of Li(BHEt3) in THF to soln. of the borane at -78°C, stirred for 5 min at room temp., volatiles removed in vac., addn. of THF, addn. of tmeda;; concentrated, washed three times with pentane, dried;; | 99% |

N,N,N',N'-Tetramethylethylenediamine Chemical Properties
Molecular formula: C6H16N2
Molar mass: 116.24 g/mol
EINECS: 203-744-6
Index of Refraction: 1.437
Density: 0.817 g/cm3
Flash Point: 10 °C
Sensitive: Hygroscopic
Melting point: -55 °C(lit.)
Storage tempreture: Store at RT.
Water solubility: Miscible
Enthalpy of Vaporization: 35.91 kJ/mol
Boiling Point: 121 °C at 760 mmHg
Vapour Pressure: 14.9 mmHg at 25 °C
Solubility: H2O: 10 mg/mL at 20 °C, clear, colorless
Appearance: Colorless to slightly yellow liquid
Structure of N,N,N',N'-Tetramethylethylenediamine (CAS NO.110-18-9):
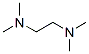
Product Category of N,N,N',N'-Tetramethylethylenediamine (CAS NO.110-18-9): Biochemistry;Reagents for Electrophoresis
N,N,N',N'-Tetramethylethylenediamine Uses
N,N,N',N'-Tetramethylethylenediamine (CAS NO.110-18-9) is widely used as a ligand for metal ions. It is used with ammonium persulfate to catalyze the polymerization of acrylamide when making polyacrylamide gels, and can be used in gel electrophoresis for the separation of proteins or nucleic acids.
N,N,N',N'-Tetramethylethylenediamine Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | oral | 630mg/kg (630mg/kg) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 28(6), Pg. 55, 1984. | |
| quail | LD50 | oral | > 316mg/kg (316mg/kg) | Ecotoxicology and Environmental Safety. Vol. 6, Pg. 149, 1982. | |
| rabbit | LD50 | skin | 5390mg/kg (5390mg/kg) | American Industrial Hygiene Association Journal. Vol. 30, Pg. 470, 1969. | |
| rabbit | LD50 | subcutaneous | 1230mg/kg (1230mg/kg) | Toxicologist. Vol. 15, Pg. 19, 1995. | |
| rat | LC50 | inhalation | 1318ppm/4H (1318ppm) | SENSE ORGANS AND SPECIAL SENSES: OTHER: EYE BEHAVIORAL: ATAXIA BEHAVIORAL: IRRITABILITY | Toxicologist. Vol. 8, Pg. 249, 1988. |
| rat | LD50 | oral | 268mg/kg (268mg/kg) | BRAIN AND COVERINGS: OTHER DEGENERATIVE CHANGES SPINAL CORD: OTHER DEGENERATIVE CHANGES | Toxicologist. Vol. 15, Pg. 19, 1995. |
N,N,N',N'-Tetramethylethylenediamine Consensus Reports
Reported in EPA TSCA Inventory.
N,N,N',N'-Tetramethylethylenediamine Safety Profile
Moderately toxic by ingestion. Mildly toxic by skin contact. A skin and severe eye irritant. Flammable when exposed to heat or flame; can react with oxidizing materials. When heated to decomposition it emits toxic fumes of NOx. See also AMINES.
Hazard Codes:  F,
F, C,
C, Xi
Xi
Risk Statements: 11-20/22-34-20/21/22
R11:Highly flammable.
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed.
R20/22:Harmful by inhalation and if swallowed.
R34:Causes burns.
Safety Statements: 16-26-36/37/39-45
S16:Keep away from sources of ignition.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
N,N,N',N'-Tetramethylethylenediamine Standards and Recommendations
DOT Classification: 3; Label: Flammable Liquid
N,N,N',N'-Tetramethylethylenediamine Specification
N,N,N',N'-Tetramethylethylenediamine , its cas register number is 110-18-9. It also can be called 1,2-Di(dimethylamino)ethane ; TMEDA ; and TEMED . It is hazardous, so the first aid measures and others should be known. Such as: When on the skin: first, should flush skin with plenty of water immediately for at least 15 minutes while removing contaminated clothing. Secondly, get medical aid. Or in the eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Then get medical aid soon. While, it's inhaled: Remove from exposure and move to fresh air immediately. Give artificial respiration while not breathing. When breathing is difficult, give oxygen. And as soon as to get medical aid. Then you have the ingesting of the product: Do not induce vomiting. If victim is conscious and alert, give 2-4 cupfuls of milk or water. Get medical aid immediately.
Related Products
- N-[(10-Oxido-9,10-dihydro-9-oxa-10-phosphaphenanthrene)methyl]-1,3,5-triazine-2,4,6-triamine
- N10-(Trifluoroacetyl)pteroic acid
- N-[1,1'-Biphenyl]-4-yl-9,9-dimethyl-9H-fluoren-2-amine
- N-[1,1'-Biphenyl]-4-yl-9,9-dimethyl-9H-fluoren-3-amine
- N-[1,1'-Biphenyl]-4-yl-N-(4-bromophenyl)-9,9-dimethyl-9H-fluoren-2-amine
- N-[1,1-Bis[(acetyloxy)methyl]-3-(4-octylphenyl)propyl]acetamide
- N'-[(1,1-Dimethylethoxy)carbonyl]-N-[(9H-fluoren-9-ylmethoxy)carbonyl]-N'-methyl-L-lysine
- N1,1-Diphenyl-1,2-ethanediamine
- N-(1,2-Dimethylpropyl)-2-pyridinamine
- N<sup xmlns="">1</sup>-(3,4-DIMETHYL-5-ISOXAZOLYL)SULFANIL-AMIDE LITHIUM SALT
- 110-19-0
- 110190-08-4
- 110192-48-8
- 110193-59-4
- 110193-60-7
- 110199-17-2
- 110201-57-5
- 110-20-3
- 11021-13-9
- 11021-14-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View