-
Name
Niclosamide
- EINECS 200-056-8
- CAS No. 50-65-7
- Article Data24
- CAS DataBase
- Density 1.616 g/cm3
- Solubility 13.32mg/L(25 oC)
- Melting Point 225-230oC
- Formula C13H8Cl2N2O4
- Boiling Point 413.905 °C at 760 mmHg
- Molecular Weight 327.124
- Flash Point 204.122 °C
- Transport Information UN 3077 9/PG 3
- Appearance white to yellowish odourless crystalline powder
- Safety 29
- Risk Codes 50
-
Molecular Structure
- Hazard Symbols
- Synonyms Fenasal;Vermitid;2,5-Dichlor-4-nitro-salizylsaeureanilid [German];Chemagro 2353;N-(2-Chloro-4-nitrophenyl)-5-chlorosalicylamide;Nitrophenyl chlorsalicylamide;Benzamide, 5-chloro-N-(2-chloro-4-nitrophenyl)-2-hydroxy-;HL 2447;Salicylanilide, 2,5-dichloro-4-nitro-, compd. with 2-aminoethanol (1:1);2, 5-Dichlor-4-nitro-salizylsaeureanilid;Mansonil;Benzamide, 5-chloro-N- (2-chloro-4-nitrophenyl)-2-hydroxy-;Lintex;Benzamide, 5-chloro-N-(2-chloro-4-nitrophenyl)-2-hydroxy-, compd with 2-aminoethanol (1:1);Vermitin;5-Chloro-N-(2-chloro-4-nitrophenyl)-2-hydroxybenzamide;Prestwick_354;Phenasal;2,5-Dichloro-4-nitrosalicylanilide;5,2-Dichloro-4-nitrosalicylanilide, ethanolamine salt;Bayer 2353;Phenasal ethanolamine salt;5-Chloro-N-(2-chloro-4-nitrophenyl)-2-hydroxybenzamide compd with 2-aminoethanol;Niclosamide [BSI:ISO];2, 5-Dichloro-4-nitrosalicylanilide;Sulqui;Niclosamide-(2-hydroxyethyl)ammonium;WR 46234;Radeverm;SR 73;B 2353;2,5-Dichloro-4-nitrosalicyloylanilide ethanolamine salt;Benzamide,5-chloro-N-(2-chloro-4- nitrophenyl)-2-hydroxy-;Niclocide (TN);1420-04-8;Helmiantin;
- PSA 95.15000
- LogP 4.45570
Synthetic route

| Conditions | Yield |
|---|---|
| With phosphorus trichloride In chlorobenzene at 135℃; for 3h; Concentration; Solvent; | 68.7% |
| With thionyl chloride In 5,5-dimethyl-1,3-cyclohexadiene at 78℃; for 6.1h; Temperature; Reflux; | |
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; | |
| With phosphorus trichloride In 5,5-dimethyl-1,3-cyclohexadiene for 4h; Reflux; |

| Conditions | Yield |
|---|---|
| With 3-(ethoxycarbonyl)-1-(5-methyl-5-(nitrosooxy)hexyl)pyridin-1-ium bis(trifluoromethanesulfonyl)imide at 20℃; for 5h; Ionic liquid; | 48% |

-
-
50-65-7
Niclosamide

| Conditions | Yield |
|---|---|
| With β-glucuronidase for 0.333333h; |

| Conditions | Yield |
|---|---|
| With dmap; N-ethyl-N,N-diisopropylamine In tetrahydrofuran; dichloromethane | |
| In dichloromethane at 25℃; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: thionyl chloride; N,N-dimethyl-formamide / dichloromethane / 12 h / 25 °C 2: dichloromethane / 25 °C / Inert atmosphere View Scheme |
-
-
50-65-7
Niclosamide

-
-
79-44-7
N,N-Dimethylcarbamoyl chloride

-
-
1187819-29-9
4-chloro-2-((2-chloro-4-nitrophenyl)carbamoyl)phenyl dimethylcarbamate

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran Reflux; | 100% |
| With pyridine; dmap Reflux; |
-
-
50-65-7
Niclosamide

| Conditions | Yield |
|---|---|
| With ammonium chloride; zinc In methanol at 0 - 20℃; for 16h; | 100% |
| With ammonium chloride; zinc In methanol; water at 0 - 20℃; for 16h; Inert atmosphere; | 100% |
| With palladium 10% on activated carbon; hydrogen In methanol; ethyl acetate at 20℃; under 3000.3 Torr; for 3h; | 99.9% |

| Conditions | Yield |
|---|---|
| With dmap; N-ethyl-N,N-diisopropylamine In tetrachloromethane; N,N-dimethyl-formamide at 0℃; for 2h; | 96.2% |
-
-
110-91-8
morpholine

-
-
50-00-0
formaldehyd

-
-
50-65-7
Niclosamide

-
-
860032-69-5
5-chloro-N-(2-chloro-4-nitrophenyl)-2-hydroxy-3-(morpholinomethyl)benzamide

| Conditions | Yield |
|---|---|
| In ethanol; water for 2h; Mannich reaction; Heating; | 95% |
| In ethanol; water Mannich reaction; Reflux; | 95% |

-
-
50-65-7
Niclosamide

-
-
74-88-4
methyl iodide

-
-
32060-31-4
5-chloro-N-(2-chloro-4-nitrophenyl)-2-methoxybenzamide

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 15h; Reflux; Sealed tube; Inert atmosphere; | 94% |
-
-
50-65-7
Niclosamide

-
-
139115-91-6
2-(2-tert-butyloxycarbonylaminoethoxy)ethanol

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 20℃; for 2h; | 93% |
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 20℃; for 2h; Mitsunobu Displacement; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| In ethanol; water for 2h; Mannich reaction; Heating; | 92% |

-
-
50-65-7
Niclosamide

-
-
77279-24-4
tert-butyl 4-(2-hydroxyethyl)piperazine-1-carboxylate

-
-
1420290-97-6
4-{2-[4-chloro-2-(2-chloro-4-nitro-phenylcarbamoyl)-phenoxy]-ethyl}-piperazine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 20℃; for 2h; | 91% |
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 20℃; for 2h; Mitsunobu Displacement; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| In ethanol; water for 2h; Mannich reaction; Heating; | 90% |


| Conditions | Yield |
|---|---|
| With dmap In N,N-dimethyl-formamide at 0℃; | 88.2% |
| With phosphoric acid at 70℃; for 1h; | 83% |
| With dmap In dichloromethane at 20℃; for 18h; |
-
-
110-89-4
piperidine

-
-
50-00-0
formaldehyd

-
-
50-65-7
Niclosamide

-
-
164589-62-2
5-chloro-N-(2-chloro-4-nitrophenyl)-2-hydroxy-3-(piperidinomethyl)benzamide

| Conditions | Yield |
|---|---|
| In ethanol; water for 2h; Mannich reaction; Heating; | 87% |
| In ethanol; water Mannich reaction; Reflux; | 87% |

-
-
371-62-0
2-fluoroethanol

-
-
50-65-7
Niclosamide

-
-
1420290-80-7
5-chloro-N-(2-chloro-4-nitro-phenyl)-2-(2-fluoro-ethoxy)benzamide

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 20℃; for 16h; Mitsunobu Displacement; Inert atmosphere; | 86% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 20℃; | 86% |
-
-
124-22-1
n-Dodecylamine

-
-
50-65-7
Niclosamide

-
-
1619232-16-4
dodecan-1-aminium 4-chloro-2-[(2-chloro-4-nitrophenyl)carbamoyl]phenoxide

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 336h; | 84% |
-
-
55750-53-3
2,5-Dihydro-2,5-dioxo-1H-pyrrole-1-hexanoic acid

-
-
50-65-7
Niclosamide

| Conditions | Yield |
|---|---|
| With triethylamine; dicyclohexyl-carbodiimide In N,N-dimethyl-formamide at 20℃; for 0.25h; Inert atmosphere; | 82% |
-
-
15159-40-7
4-morpholinocarbonyl chloride

-
-
50-65-7
Niclosamide

-
-
1243092-32-1
4-chloro-2-((2-chloro-4-nitrophenyl)carbamoyl)phenyl morpholine-4-carboxylate

| Conditions | Yield |
|---|---|
| With pyridine at 60℃; | 81% |
| With pyridine; dmap for 3h; Reflux; |
-
-
143-27-1
hexadecylamine

-
-
50-65-7
Niclosamide

-
-
1619232-17-5
hexadecan-1-aminium 4-chloro-2-[(2-chloro-4-nitrophenyl)carbamoyl]phenoxide

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 336h; | 81% |
-
-
50-65-7
Niclosamide

-
-
23238-76-8
5-chloro-N-(2-chloro-4-nitrophenyl)-2-hydroxybenzenecarbothioamide

| Conditions | Yield |
|---|---|
| With Lawessons reagent In acetonitrile for 24h; Reflux; | 80% |
-
-
50-65-7
Niclosamide

-
-
77544-68-4
8-tosyloxy-3,6-dioxaoctanol

-
-
1046321-41-8
O-(2-[2-(2-hydroxyethoxy)ethoxy]ethyl)niclosamide

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; potassium carbonate In 1,4-dioxane at 79.99℃; for 12h; | 79% |
| With tetrabutylammomium bromide; potassium carbonate In 1,4-dioxane at 85℃; for 6h; Reflux; | 78% |

| Conditions | Yield |
|---|---|
| Stage #1: isocyanate de chlorosulfonyle With formic acid at 0℃; Stage #2: Niclosamide With pyridine In dichloromethane at 50℃; for 6h; | 76% |

| Conditions | Yield |
|---|---|
| With pyridine In chloroform at 0℃; for 3h; | 75% |
-
-
50-65-7
Niclosamide

-
-
540-51-2
2-bromoethanol

-
-
1046321-35-0
2-(2-bromoethoxy)-5-chloro-N-(2-chloro-4-nitrophenyl)benzamide

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 20℃; for 16h; Mitsunobu Displacement; Inert atmosphere; | 75% |

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 337.92h; | 74% |
-
-
26690-80-2
2-(N-tert-butoxycarbonylamino)ethanol

-
-
50-65-7
Niclosamide

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 20℃; | 71% |
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran | 45% |
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 20℃; for 2h; Mitsunobu Displacement; Inert atmosphere; |
-
-
50-65-7
Niclosamide

-
-
77544-60-6
p-toluenesulfonic acid 2-[2-[2-(2-hydroxyethoxy)ethoxy]ethoxy]ethyl ester

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; potassium carbonate In 1,4-dioxane at 79.99℃; for 12h; | 70% |

| Conditions | Yield |
|---|---|
| In ethanol; water 1.) reflux, 5 h, 2.) r.t., overnight; | 65% |

-
-
50-65-7
Niclosamide

| Conditions | Yield |
|---|---|
| Stage #1: Niclosamide With N-ethyl-N,N-diisopropylamine In acetonitrile Stage #2: With potassium fluoride; N,N`-sulfuryldiimidazole; trifluoroacetic acid In water at 20℃; for 18h; | 64% |
-
-
50-65-7
Niclosamide

| Conditions | Yield |
|---|---|
| With rhodium(III) chloride; water-d2 In N,N-dimethyl-formamide at 108℃; | 60% |
Niclosamide Chemical Properties
IUPAC Name: 5-chloro-N-(2-chloro-4-nitrophenyl)-2-hydroxybenzamide
Empirical Formula: C13H8Cl2N2O4
Molecular Weight: 327.1196g/mol
EINECS: 200-056-8
Structure of Niclosamide (CAS NO.50-65-7):
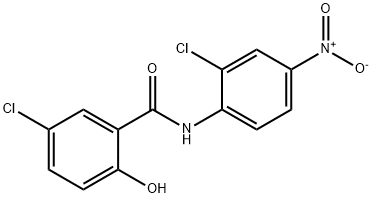
Index of Refraction: 1.709
Molar Refractivity: 79.04 cm3
Molar Volume: 202.4 cm3
Polarizability: 31.33×10-24cm3
Surface Tension: 71.6 dyne/cm
Density: 1.615 g/cm3
Flash Point: 210.5 °C
Enthalpy of Vaporization: 70.52 kJ/mol
Boiling Point: 424.5 °C at 760 mmHg
Vapour Pressure: 8.35E-08 mmHg at 25°C
Product Categories: Veterinaries;API's
Canonical SMILES: C1=CC(=C(C=C1[N+](=O)[O-])Cl)NC(=O)C2=C(C=CC(=C2)Cl)O
InChI: InChI=1S/C13H8Cl2N2O4/c14-7-1-4-12(18)9(5-7)13(19)16-11-3-2-8(17(20)21)6-10(11)15/h1-6,18H,(H,16,19)
InChIKey: RJMUSRYZPJIFPJ-UHFFFAOYSA-N
Niclosamide Uses
Niclosamide (CAS NO.50-65-7) is used as a piscicide.It is stressed that while anthelmintics are a drug family used to treat worm infections, Niclosamide is used specifically to treat tapeworms and is not effective against worms such as pinworms or roundworms.
Niclosamide Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| cat | LD | oral | > 1gm/kg (1000mg/kg) | French Medicament Patent Document. Vol. #2628M, | |
| mouse | LD50 | intravenous | 7500ug/kg (7.5mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Arzneimittel-Forschung. Drug Research. Vol. 10, Pg. 884, 1960. |
| mouse | LD50 | oral | 1gm/kg (1000mg/kg) | "Wirksubstanzen der Pflanzenschutz und Schadlingsbekampfungsmittel," Perkow, W., Berlin, Verlag Paul Parey, 1971-1976Vol. -, Pg. -, 1971/1976. | |
| rabbit | LD | oral | > 5gm/kg (5000mg/kg) | French Medicament Patent Document. Vol. #2628M, | |
| rat | LD50 | intraperitoneal | 250mg/kg (250mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Zeitschrift fuer Tropenmedizin und Parasitologie. Vol. 13, Pg. 1, 1962. |
| rat | LD50 | oral | 2500mg/kg (2500mg/kg) | "Agrochemicals Handbook," with updates, Hartley, D., and H. Kidd, eds., Nottingham, Royal Soc of Chemistry, 1983-86Vol. A297, Pg. 1983, |
Niclosamide Safety Profile
RIDADR: UN 3077 9/PG 3
WGK Germany: 2
RTECS: VN8400000
Niclosamide Specification
Niclosamide , its cas register number is 50-65-7. It also can be called 2',5-Dichlor-4'-nitro-salizylsaeureanilid ; 2',5-Dichloro-4'-nitrosalicylanilide ; 2-Chloro-4-nitrophenylamide-6-chlorosalicylic acid ; 2-Hydroxy-5-chloro-N-(2-chloro-4-nitrophenyl)benzamide ; 5-Chloro-2'-chloro-4'-nitrosalicylanilide ; 5-Chloro-N-(2-chloro-4-nitrophenyl)-2-hydroxybenzamide ; 5-Chlorosalicyloyl-(o-chloro-p-nitranilide) ; Atenase ; Bayluscid ; Cestocid ; Devermine ; Dichlosale ; Fedal-Telmin ; Fenasal ; Helmiantin ; Lintex ; Mansonil ; Mato ; N-(2'-Chlor-4'-nitrophenyl)-5-chlorsalicylamid ; Nasemo ; Niclosamida ; Niclosamide ; Niclosamidum ; Nitrophenyl chlorsalicylamide ; Sagimid ; Salicylanilide, 2',5-dichloro-4'-nitro- ; Sulqui ; Vermitid ; Vermitin ;Yomesan ; Zestocarp .
Related Products
- Niclosamide
- Niclosamide ethanolamine salt
- Niclosamide piperazine salt
- 506-59-2
- 506-61-6
- 50662-99-2
- 50663-21-3
- 5066-34-2
- 50663-54-2
- 506-63-8
- 506-64-9
- 50667-33-9
- 50-66-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View