-
Name
Octanal
- EINECS 204-683-8
- CAS No. 124-13-0
- Article Data514
- CAS DataBase
- Density 0.821 g/cm3
- Solubility Slightly soluble in water
- Melting Point 12-15 °C(lit.)
- Formula C8H16O
- Boiling Point 194.665 °C at 760 mmHg
- Molecular Weight 128.214
- Flash Point 81.111 °C
- Transport Information UN 1191 3/PG 3
- Appearance liquid
- Safety 16
- Risk Codes 10
-
Molecular Structure
- Hazard Symbols R10:;
- Synonyms AldehydeC 8;Antifoam LF;Caprylaldehyde;Caprylic aldehyde;NSC 1508;NSC 8969;Octaldehyde;Octanaldehyde;Octanoic aldehyde;Octylaldehyde;n-Caprylaldehyde;n-Octaldehyde;n-Octanal;n-Octyl aldehyde;n-Octylal;
- PSA 17.07000
- LogP 2.54580
Synthetic route

| Conditions | Yield |
|---|---|
| With methanesulfonic acid; tributylphosphine; copper; zinc In acetonitrile for 1h; Product distribution; Ambient temperature; reactions of other acid chlorides; solvent-effect; effect of var. metals; | 100% |
| With tri-n-butyl-tin hydride In 1-methyl-pyrrolidin-2-one at 20℃; Inert atmosphere; | 96% |
| With pumice stone; platinum at 195℃; under 80 - 90 Torr; Hydrogenation; |
-
-
155127-38-1
((E)-4-Oct-1-enyl)-morpholine

-
-
124-13-0
Octanal

| Conditions | Yield |
|---|---|
| With aq. acid for 1h; Ambient temperature; | 100% |
-
-
59092-72-7
2-heptyl-[1,3]dithiane

-
-
124-13-0
Octanal

| Conditions | Yield |
|---|---|
| With silica gel; ferric nitrate In hexane at 50℃; for 0.166667h; | 100% |
| With silica gel; copper(II) nitrate In tetrachloromethane for 0.416667h; Ambient temperature; | 98% |
| With dihydrogen peroxide; tantalum pentachloride; sodium iodide In water; ethyl acetate at 20℃; for 68h; | 85% |

| Conditions | Yield |
|---|---|
| With chromium (VI) oxide In toluene for 0.416667h; microwave irradiation; | 99% |
| With N-chloro-succinimide; N-(phenylthio)-N-(tert-butyl)amine; potassium carbonate; 4 A molecular sieve In dichloromethane at 20℃; for 3h; | 99% |
| With 2,2,6,6-tetramethyl-piperidine-N-oxyl; oxygen; copper(I) bromide dimethylsulfide complex In chlorobenzene at 80℃; for 8h; | 99% |
-
-
111-87-5
octanol

-
-
33524-31-1
2,5-dimethoxybenzyl alcohol

-
A
-
124-13-0
Octanal

-
B
-
93-02-7
2,5-dimethoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With air; potassium carbonate at 20℃; for 16h; | A 2% B 99% |

-
-
93215-67-9
octanal 1,3-dithiolane

-
-
124-13-0
Octanal

| Conditions | Yield |
|---|---|
| With silica gel; copper(II) nitrate In tetrachloromethane for 0.25h; Ambient temperature; | 98% |
| With silica gel; ferric nitrate In hexane at 50℃; for 0.166667h; | 96% |
| With bismuth(III) nitrate; water In benzene at 20℃; for 4h; | 98 % Chromat. |

| Conditions | Yield |
|---|---|
| With (acetylacetonato)dicarbonylrhodium (l); C43H53O8P; hydrogen In toluene under 37503.8 Torr; for 12h; Catalytic behavior; regioselective reaction; | 97.1% |
| With Ca8(PO4)3.5(HPO4)2.5(OH)0.5; hydrogen; Rh2(μ-S-t-Bu)2(CO)2(P(C6H4SO3Na)3)2 In toluene at 80℃; under 3750.38 Torr; for 3h; Product distribution; Further Variations:; Reagents; hydration rate of reagent; | 93% |

| Conditions | Yield |
|---|---|
| With silica gel; copper(II) nitrate In tetrachloromethane for 1.66667h; Heating; | 97% |
| With ammonium peroxydisulfate; Montmorillonite K10; silver nitrate In hexane at 50℃; for 2.5h; | 94% |
| With water; Dess-Martin periodane In dichloromethane at 5℃; for 0.5h; | 92% |
-
-
69873-64-9
4-methyl-N'-octylidenebenzene-1-sulfonohydrazide

-
-
124-13-0
Octanal

| Conditions | Yield |
|---|---|
| With silica gel; copper(II) nitrate In tetrachloromethane for 2h; Heating; | 97% |

| Conditions | Yield |
|---|---|
| With 5-ethyl-2-methyl-pyridine; 4-acetylamino-2,2,6,6-tetramethylpiperidine-N-oxyl; iodine; sodium hydrogencarbonate In dichloromethane for 1h; Reagent/catalyst; | A 96.6% B 3.4% |
| With 2,2,6,6-tetramethyl-piperidine-N-oxyl; tert.-butylnitrite; oxygen In 1,2-dichloro-ethane under 1500.15 Torr; for 6h; Autoclave; Heating; | A 94% B 1% |
| With water; oxygen at 100℃; under 3750.38 Torr; for 18h; Autoclave; | A 9% B 91% |

| Conditions | Yield |
|---|---|
| With sodium dodecyl-sulfate; [Ru(η5-C9H7(Cl(PPh3)2] In water at 60℃; for 24h; | 95% |
| With chloro(cyclopentadienyl)[bis(diphenylphosphino)methane]ruthenium; water In isopropyl alcohol at 100℃; for 12h; | 93% |
| With borane N,N-diethylaniline complex; dihydrogen peroxide; sodium acetate; benzo[1,3,2]dioxaborole 1.) benzene, 25 deg C, 24 h; Yield given. Multistep reaction; |
-
-
14246-16-3
trimethyl(oct-1-yloxy)silane

-
-
124-13-0
Octanal

| Conditions | Yield |
|---|---|
| With n-butyltriphenylphosphonium peroxodisulfate In acetonitrile for 0.5h; Heating; | 95% |
| With chromium(VI) oxide; HZSM-5 zeolite for 0.025h; microwave irradiation; | 93% |
| With chromium(VI) oxide; Hexamethyldisiloxane; silica gel In dichloromethane for 0.333333h; Ambient temperature; | 89% |

| Conditions | Yield |
|---|---|
| Stage #1: n-Octylamine With tungsten(IV) sulfide at 46℃; for 3h; Stage #2: With isobutyl formate for 1.83333h; Temperature; Reflux; | 94% |
| With potassium hydroxide In ethyl acetate at -78℃; Product distribution; | 39% |
| Multi-step reaction with 3 steps 1: 98 percent / Et3N / CH2Cl2 / 1 h / 0 °C 2: N-tert-butylphenylsulfinimidoyl chloride; DBU / CH2Cl2 / 1 h / -78 °C 3: HCl; H2O / diethyl ether; CH2Cl2 / 2 h / 50 °C View Scheme |

| Conditions | Yield |
|---|---|
| With phosphate buffer; D-glucose; Synechococcus sp. PCC 7942; Triton X-100 In water at 25℃; for 72h; pH=7.0; Irradiation; | A 94% B 5% |
-
-
122744-71-2
trans-Dihydro-3-phenyl-5-(phenylmethyl)-6-heptyl-1,2,4,5-trioxazine

-
A
-
124-13-0
Octanal

-
B
-
72552-76-2
α-Heptyl-N-benzylnitrone

-
C
-
65-85-0
benzoic acid

-
D
-
932-90-1
Benzaldoxime

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol for 24h; Ambient temperature; other base; | A 18% B 49% C 93% D 20% |

-
-
23162-72-3
octylidene diacetate

-
-
124-13-0
Octanal

| Conditions | Yield |
|---|---|
| With H6P2W18O62 In toluene at 100℃; for 0.0833333h; | 93% |
| With sodium tetrahydroborate; nickel(II) chloride hexahydrate In methanol at 20℃; for 3.5h; chemoselective reaction; | 86% |
-
-
2548-87-0, 20664-46-4, 2363-89-5
2-octen-1-al

-
-
124-13-0
Octanal

| Conditions | Yield |
|---|---|
| With acetylacetonatodicarbonylrhodium(l); trifluorormethanesulfonic acid; carbon monoxide; N-(5-diphenylphosphanylpyrrole-2-carbonyl)guanidine; hydrogen In dichloromethane at 40℃; under 15001.5 Torr; for 3h; Autoclave; chemoselective reaction; | 93% |
| With hydrogen; tetra-(n-butyl)ammonium iodide In water at 110℃; under 15001.5 Torr; for 24h; chemoselective reaction; | 82% |
| With dibenzylammonium trifluoroacetate salt In dichloromethane at 20℃; for 24h; | 82 %Chromat. |
| With hydrogen; sodium dodecyl-sulfate; palladium diacetate In tetrahydrofuran; water at 20℃; under 750.075 Torr; for 1h; Reagent/catalyst; chemoselective reaction; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; sodium nitrite In dichloromethane; water at 20℃; under 760.051 Torr; for 12h; in air; | A 92% B 5.2% |
| With Succinimide; sodium hypochlorite solution; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; potassium carbonate In ethyl acetate at 0 - 10℃; for 2h; | A 86% B 7% |
| With potassium chromate; copolyesteramide (from N,N'-bis(4-methoxycarbonylbenzoyl)hexamethylenediamine, 1,6-hexanediol, poly(ethylene glycol)); sulfuric acid In dichloromethane at -5℃; for 0.25h; | A 83% B 2.1% |
-
-
10022-28-3
1,1-dimethoxyoctane

-
-
124-13-0
Octanal

| Conditions | Yield |
|---|---|
| indium(III) chloride In methanol; water for 1.16667h; Heating; | 92% |
| 1,3-di(NCS)-tetrabutyldistannoxane In diethylene glycol dimethyl ether; water at 100℃; for 2h; Deprotection of acetal; | 85% |
| With titanium tetrachloride; lithium iodide In diethyl ether for 3h; Product distribution; Ambient temperature; | 85% |
| Tetrabutyl-1,3-diisothiocyanato-distannoxane In diethylene glycol dimethyl ether; water at 100℃; for 2h; | 85% |
-
-
35066-22-9
2-bromooctanal

-
-
124-13-0
Octanal

| Conditions | Yield |
|---|---|
| With DMBI In tetrahydrofuran for 5h; Heating; | 92% |
-
-
13175-44-5
oct-7-en-1-ol

-
-
124-13-0
Octanal

| Conditions | Yield |
|---|---|
| With potassium hydroxide; PS-resin; potassium hexacyanoferrate(III); 4-(benzyloxycarbonyl)-2,2,6,6-tetramethylpiperidine-1-oxyl In water; toluene at 20℃; for 24h; | 92% |
-
-
99178-22-0
N'-octylidene-N,N-dimethyl-hydrazine

-
-
124-13-0
Octanal

| Conditions | Yield |
|---|---|
| With Glyoxilic acid In water at 20℃; for 4h; | 91% |
| tin(ll) chloride; palladium dichloride In water for 0.025h; microwave irradiation; | 88% |

| Conditions | Yield |
|---|---|
| indium(III) chloride In methanol; water for 1.33333h; Heating; | 90% |
| 1,3-di(NCS)-tetrabutyldistannoxane In diethylene glycol dimethyl ether; water at 100℃; for 2h; Deprotection of acetal; | 87% |
| Tetrabutyl-1,3-diisothiocyanato-distannoxane In diethylene glycol dimethyl ether; water at 100℃; for 2h; | 87% |

| Conditions | Yield |
|---|---|
| With Li(1+)*C12H28AlO3(1-) In tetrahydrofuran; hexane for 1h; Ambient temperature; Yield given; | A n/a B 90% |
| With Li(1+)*C12H28AlO3(1-) In tetrahydrofuran; hexane for 1h; Ambient temperature; Yields of byproduct given; | A n/a B 90% |

| Conditions | Yield |
|---|---|
| With Li(1+)*C12H28AlO3(1-) In tetrahydrofuran; hexane for 1h; Ambient temperature; Yields of byproduct given; | A n/a B 90% |
| With Li(1+)*C12H28AlO3(1-) In tetrahydrofuran; hexane for 0.5h; Ambient temperature; Yield given. Yields of byproduct given. Title compound not separated from byproducts; |
-
-
3240-34-4
[bis(acetoxy)iodo]benzene

-
-
63318-32-1
3-methylthio-5-octyl-1,4-diphenyl-1,2,4-triazolium iodide

-
A
-
591-50-4
iodobenzene

-
B
-
124-13-0
Octanal

-
C
-
13136-15-7
1,4-Diphenyl-3-methylmercapto-1,2,4-triazolium iodide

| Conditions | Yield |
|---|---|
| With sulfuric acid; iodine; sodium methylate; potassium iodide 1.) CHCl3, 2.) MeOH, room temp, 15 min; Yield given. Multistep reaction; | A 90% B n/a C 31% |


| Conditions | Yield |
|---|---|
| With dimethyl sulfoxide for 0.0638889h; Kornblum oxidation; Microwave irradiation; | 89% |
| With sodium periodate In N,N-dimethyl-formamide at 150℃; for 0.75h; | 85% |
| With sodium hydrogencarbonate; dimethyl sulfoxide; sodium iodide at 115℃; for 2h; | 60% |
| With silver(I) 4-methylbenzenesulfonate; acetonitrile Erhitzen mit Dimethylsulfoxyd und Natriumhydrogencarbonat auf 150grad; | |
| With 4-(dimethylamino)pyridine N-oxide; 1,8-diazabicyclo[5.4.0]undec-7-ene 1.) CH3CN, reflux, 15 min, 2.) CH3CN, reflux, 40 min; Yield given. Multistep reaction; |
-
-
122744-71-2
trans-Dihydro-3-phenyl-5-(phenylmethyl)-6-heptyl-1,2,4,5-trioxazine

-
A
-
124-13-0
Octanal

-
B
-
72552-76-2
α-Heptyl-N-benzylnitrone

-
C
-
100-52-7
benzaldehyde

| Conditions | Yield |
|---|---|
| With titanium tetrachloride In dichloromethane for 0.5h; Product distribution; Ambient temperature; | A 44% B 28% C 89% |

-
-
54852-64-1
((octyloxy)methyl)benzene

-
-
124-13-0
Octanal

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; chromium-pillared montmorillonite In 2,2,4-trimethylpentane; dichloromethane for 18h; Ambient temperature; | 88% |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran; butan-1-ol at 23℃; Inert atmosphere; | 100% |
| With silica gel; triethylamine at 20℃; for 3h; Henry Nitro Aldol Condensation; | 95% |
| With potassium tert-butylate In tetrahydrofuran; tert-butyl alcohol at 20℃; for 18.5h; Henry reaction; | 94% |

| Conditions | Yield |
|---|---|
| With lithium tetrafluoroborate In methanol for 0.5h; Heating; | 100% |
| With cerium triflate In methanol at 20℃; for 0.0166667h; | 95% |
| With Pd(PhCN)2(OTf)2 at 20℃; for 0.333333h; Inert atmosphere; | 90% |

| Conditions | Yield |
|---|---|
| With n-butyllithium In 1,2-dimethoxyethane; hexane 1.) 0 deg C, 10 min, 2.) 30 min; | 100% |
| Stage #1: diethoxyphosphoryl-acetic acid ethyl ester With sodium hydride In diethyl ether at 0℃; for 0.0833333h; Stage #2: Octanal In diethyl ether for 0.25h; | 96% |
| Stage #1: diethoxyphosphoryl-acetic acid ethyl ester With sodium hydride In diethyl ether; mineral oil at 0℃; Stage #2: Octanal In diethyl ether; mineral oil at 0℃; | 96% |
-
-
124-13-0
Octanal

-
-
59983-39-0
(S)-1-amino-2-(methoxymethyl)pyrrolidine

-
-
72170-92-4
((S)-2-Methoxymethyl-pyrrolidin-1-yl)-oct-(E)-ylidene-amine

| Conditions | Yield |
|---|---|
| at 20℃; for 16h; | 100% |
| Yield given; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In tetrahydrofuran at 20℃; for 1h; | 100% |
| With C20H23BBiF4N In methanol; water at 20℃; for 1h; | 95% |
| With C17H18BiF3O5S In methanol at 20℃; for 1h; | 92% |
-
-
124-13-0
Octanal

-
-
593-51-1
methylamine hydrochloride

-
-
151-50-8
potassium cyanide

-
-
112101-12-9
2-Methylamino-nonanenitrile

| Conditions | Yield |
|---|---|
| With aluminum oxide In acetonitrile at 50℃; for 22h; ultrasound; | 100% |


| Conditions | Yield |
|---|---|
| With hydrogen; Et4N In 1,2-dimethoxyethane at 100℃; under 38000 Torr; for 13h; | 100% |
| With zirconium(IV) tetraisopropoxide 2-propanol; 4 Angstroem MS; 1,1'-bi-2-naphthol In isopropyl alcohol; toluene at 20℃; for 18h; | 100% |
| With zirconium(IV) tetraisopropoxide 2-propanol; 1,1'-bi-2-naphthol In toluene at 20℃; for 1h; | 100% |

| Conditions | Yield |
|---|---|
| With tributylphosphine; 1,1'-bi-2-naphthol In tetrahydrofuran at 20℃; for 3h; Baylis-Hillman addition; | 100% |

| Conditions | Yield |
|---|---|
| 100% |

| Conditions | Yield |
|---|---|
| With 4-cyanobenzaldehyde In Petroleum ether at 20℃; for 16h; Reagent/catalyst; Sealed tube; Irradiation; | 100% |
| With dibenzoyl peroxide | |
| With dibenzoyl peroxide |

| Conditions | Yield |
|---|---|
| With water; sodium sulfate at 20℃; for 7h; Passerini Condensation; | 100% |
| With air; water at 40℃; for 3h; | 71% |

| Conditions | Yield |
|---|---|
| With Et4NF*5HF at 20℃; for 0.166667h; Prins reaction; stereoselective reaction; | 100% |
| With Et4NF*5HF at 20℃; for 0.166667h; Prins cyclization; stereoselective reaction; |

| Conditions | Yield |
|---|---|
| With Et4NF*5HF at 20℃; for 0.166667h; Prins reaction; stereoselective reaction; | 100% |
| With Et4NF*5HF at 20℃; for 0.166667h; Prins cyclization; stereoselective reaction; |

| Conditions | Yield |
|---|---|
| With potassium carbonate In dichloromethane at 40℃; for 24h; Wittig reaction; Inert atmosphere; optical yield given as %de; diastereoselective reaction; | 100% |

| Conditions | Yield |
|---|---|
| In methanol for 3h; Reflux; | 100% |

| Conditions | Yield |
|---|---|
| With water; sodium sulfate at 20℃; for 7h; Passerini Condensation; | 100% |

| Conditions | Yield |
|---|---|
| Reagent/catalyst; Aldol Condensation; Inert atmosphere; | 100% |
-
-
124-13-0
Octanal

| Conditions | Yield |
|---|---|
| With sodium cyanoborohydride In ethanol; water; acetic acid at 40℃; for 1h; Microwave irradiation; | 100% |
-
-
124-13-0
Octanal

| Conditions | Yield |
|---|---|
| With sodium cyanoborohydride In ethanol; water; acetic acid at 40℃; for 1h; Temperature; Time; Microwave irradiation; | 100% |
-
-
124-13-0
Octanal

| Conditions | Yield |
|---|---|
| With sodium cyanoborohydride In ethanol; water; acetic acid at 40℃; for 1h; Temperature; Time; Microwave irradiation; | 100% |
-
-
124-13-0
Octanal

| Conditions | Yield |
|---|---|
| With sodium cyanoborohydride In ethanol; water; acetic acid at 40℃; for 1h; Microwave irradiation; | 100% |
-
-
124-13-0
Octanal

| Conditions | Yield |
|---|---|
| With sodium cyanoborohydride In ethanol; water; acetic acid at 40℃; for 1h; Temperature; Time; Microwave irradiation; | 100% |
-
-
124-13-0
Octanal

| Conditions | Yield |
|---|---|
| With sodium cyanoborohydride In ethanol; water; acetic acid at 40℃; for 1h; Temperature; Time; Microwave irradiation; | 100% |
-
-
124-13-0
Octanal

-
-
1354483-32-1
(1R)-1-C-allyl-2,3,4-tri-O-benzyl-1,5-dideoxy-1,5-imino-D-xylitol

| Conditions | Yield |
|---|---|
| With sodium cyanoborohydride; acetic acid In ethanol at 20℃; for 6h; | 100% |

| Conditions | Yield |
|---|---|
| With sodium butanolate In butan-1-ol Reflux; | 100% |
-
-
124-13-0
Octanal

-
-
13086-95-8
1,1-diacetoxy-1-(2-chlorophenyl)methane

| Conditions | Yield |
|---|---|
| With 2-(4-methoxyphenyl)-6,7-dihydro-5H-pyrrolo[2,1-c][1,2,4]triazol-2-ium tetrafluoroborate; potassium carbonate In tetrahydrofuran for 24h; Inert atmosphere; Reflux; | 100% |
-
-
124-13-0
Octanal

-
-
944883-07-2
[2-methyl-2-phenyl-1,3-oxazolidin-4-yl]methanol

| Conditions | Yield |
|---|---|
| 99.9% |
Octanal Chemical Properties
MF: C8H16O
MW: 128.21
EINECS: 204-683-8
mp: 12-15 °C(lit.)
bp: 171 °C(lit.)
density: 0.822 g/mL at 20 °C
vapor pressure: 2 mm Hg ( 20 °C)
refractive index: n20/D 1.421(lit.)
FEMA: 2797
Fp: 125 °F
storage temp: 0-6°C
Water Solubility : slightly soluble
Merck: 14,1766
The structure of Octanal is:
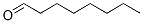
Octanal Uses
Octanal Toxicity Data With Reference
| 1. | skn-rbt 500 mg/24H MLD | FCTXAV Food and Cosmetics Toxicology. 11 (1973),1079. | ||
| 2. | eye-rbt 100 mg MLD | FCTXAV Food and Cosmetics Toxicology. 11 (1973),1079. | ||
| 3. | orl-rat LD50:5630 mg/kg | FCTXAV Food and Cosmetics Toxicology. 11 (1973),95. | ||
| 4. | skn-rbt LD50:6350 mg/kg | FCTXAV Food and Cosmetics Toxicology. 11 (1973),95. |
Octanal Consensus Reports
Octanal Safety Profile
Risk Statements: 10
Safety Statements: 16
RIDADR: UN 1191 3/PG 3
WGK Germany: 2
RTECS: RG7780000
F: 10
HazardClass: 3
PackingGroup: III
Mildly toxic by ingestion and skin contact. A skin and eye irritant. Flammable liquid when exposed to heat, sparks, or flame. Can react with oxidizing materials. To fight fire, use foam, CO2, dry chemical.
Octanal Specification
1、Fire Fighting Measures
General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Will burn if involved in a fire. Flammable liquid and vapor.
Extinguishing Media: In case of fire, use water, dry chemical, chemical foam, or alcohol-resistant foam. Use agent most appropriate to extinguish fire.
2、Handling and Storage
Handling: Use spark-proof tools and explosion proof equipment. Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes. Empty containers retain product residue, (liquid and/or vapor), and can be dangerous. Keep away from heat, sparks and flame.
Storage: Keep away from heat, sparks, and flame. Keep away from sources of ignition. Store in a tightly closed container. Keep refrigerated. (Store below 4°C/39°F.)
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View