-
Name
Osthole
- EINECS 1532714-185-1
- CAS No. 484-12-8
- Article Data11
- CAS DataBase
- Density 1.126 g/cm3
- Solubility 12mg/L(25 oC)
- Melting Point 83-84 °C
- Formula C15H16O3
- Boiling Point 396.7 °C at 760 mmHg
- Molecular Weight 244.29
- Flash Point 167.6 °C
- Transport Information
- Appearance Fine White Powder
- Safety
- Risk Codes R36/37/38; R20/21/22
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi, Xn
Xn
- Synonyms 8-(3-Methyl-2-butenyl)herniarin;2H-1-Benzopyran-2-one, 7-methoxy-8-(3-methyl-2-butenyl)-;7-Methoxy-8-isopentenylcoumarin;7-methoxy-8-(3-methylbut-2-enyl)chromen-2-one;Coumarin, 7-methoxy-8- (3-methyl-2-butenyl)-;2H-1-Benzopyran-2-one,7-methoxy-8-(3- methyl-2-butenyl)-;Ostol;Coumarin, 7-methoxy-8-(3-methyl-2-butenyl)-;Cnidium monnieri P.E.- Osthole;Common Cnidium Fruit P. E.;Fructus Cnidii;
- PSA 39.44000
- LogP 3.31030
Synthetic route

| Conditions | Yield |
|---|---|
| Stage #1: 7-methoxy-8-iodo-2H-chromen-2-one With isopropylmagnesium chloride In tetrahydrofuran at -20℃; for 1h; Schlenk technique; Inert atmosphere; Green chemistry; Stage #2: prenyl bromide With copper(l) iodide; lithium chloride In tetrahydrofuran at -20 - 20℃; for 10.5h; Reagent/catalyst; Schlenk technique; Inert atmosphere; Green chemistry; | 80% |

| Conditions | Yield |
|---|---|
| With tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride In dichloromethane at 45℃; for 2h; Inert atmosphere; | 77.65% |

| Conditions | Yield |
|---|---|
| With N,N-diethylaniline at 250℃; for 1h; Microwave irradiation; Sealed tube; | 68% |

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); triphenylphosphine; lithium chloride In N,N-dimethyl-formamide at 80℃; for 6h; Stille Cross Coupling; Inert atmosphere; Schlenk technique; | 66.1% |

| Conditions | Yield |
|---|---|
| With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride In N,N-dimethyl-formamide at 125℃; for 5h; Stille Cross Coupling; Schlenk technique; Inert atmosphere; | 32% |
-
-
37761-53-8
2-hydroxy-4-methoxy-3-(3-methyl-2-butenyl)benzaldehyde

-
-
108-24-7
acetic anhydride

-
-
484-12-8
osthole

| Conditions | Yield |
|---|---|
| With sodium acetate at 160℃; |

| Conditions | Yield |
|---|---|
| With diethyl ether |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium; benzene / anschliessend mit 4-Brom-2-methyl-buten-(2) 2: sodium acetate / 160 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: aluminum (III) chloride / dichloromethane / 0.25 h / -20 °C 1.2: -20 - 20 °C 2.1: sodium hydride / tetrahydrofuran; mineral oil / 1.5 h / Reflux 2.2: 12 h / 0 °C 3.1: dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer; copper(II) acetate monohydrate / acetonitrile / 16 h / 85 °C 4.1: (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride / N,N-dimethyl-formamide / 5 h / 125 °C / Schlenk technique; Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: potassium carbonate; potassium iodide / acetone / 22 h / 60 °C 2: ethylene glycol / 6 h / Reflux 3: potassium carbonate; potassium iodide / acetone / 5 h / 20 °C 4: tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride / dichloromethane / 2 h / 45 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1.1: iodine; potassium iodide; ammonium hydroxide / water / 24.5 h 2.1: potassium carbonate / acetone / 5 h / Reflux 3.1: isopropylmagnesium chloride / tetrahydrofuran / 1 h / -20 °C / Schlenk technique; Inert atmosphere; Green chemistry 3.2: 10.5 h / -20 - 20 °C / Schlenk technique; Inert atmosphere; Green chemistry View Scheme |
-
-
55136-72-6
7-hydroxy-8-allylcoumarin

-
-
484-12-8
osthole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: potassium carbonate; potassium iodide / acetone / 5 h / 20 °C 2: tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride / dichloromethane / 2 h / 45 °C / Inert atmosphere View Scheme |
-
-
31005-03-5
7-allyloxycoumarin

-
-
484-12-8
osthole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: ethylene glycol / 6 h / Reflux 2: potassium carbonate; potassium iodide / acetone / 5 h / 20 °C 3: tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride / dichloromethane / 2 h / 45 °C / Inert atmosphere View Scheme |
-
-
17577-28-5
(ethoxycarbonylmethyl)triphenylphosphonium chloride

-
-
484-12-8
osthole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: potassium hydroxide / ethanol / 1.5 h / 80 °C 2: potassium carbonate; potassium iodide / acetone / 22 h / 60 °C 3: ethylene glycol / 6 h / Reflux 4: potassium carbonate; potassium iodide / acetone / 5 h / 20 °C 5: tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride / dichloromethane / 2 h / 45 °C / Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: ethanol / 4 h / 80 °C 2: potassium hydroxide / ethanol / 1.5 h / 80 °C 3: potassium carbonate; potassium iodide / acetone / 22 h / 60 °C 4: ethylene glycol / 6 h / Reflux 5: potassium carbonate; potassium iodide / acetone / 5 h / 20 °C 6: tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride / dichloromethane / 2 h / 45 °C / Inert atmosphere View Scheme |
-
-
65763-00-0
7-hydroxy-8-iodocoumarin

-
-
484-12-8
osthole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: potassium carbonate / acetone / 5 h / Reflux 2.1: isopropylmagnesium chloride / tetrahydrofuran / 1 h / -20 °C / Schlenk technique; Inert atmosphere; Green chemistry 2.2: 10.5 h / -20 - 20 °C / Schlenk technique; Inert atmosphere; Green chemistry View Scheme |

-
-
21204-67-1
methyl (triphenylphosphoranylidene)acetate

-
-
484-12-8
osthole

| Conditions | Yield |
|---|---|
| In toluene at 220℃; for 1h; Inert atmosphere; Microwave irradiation; Sealed tube; | 928 mg |

-
-
484-12-8
osthole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: hydrogen / ethyl acetate / 12 h / 20 °C / 760.05 Torr 2: toluene / 1 h / 220 °C / Inert atmosphere; Microwave irradiation; Sealed tube View Scheme |
-
-
63638-85-7
3-bromo-2-hydroxy-4-methoxybenzaldehyde

-
-
484-12-8
osthole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: sodium hydride / tetrahydrofuran; mineral oil / 1.5 h / Reflux 1.2: 12 h / 0 °C 2.1: dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer; copper(II) acetate monohydrate / acetonitrile / 16 h / 85 °C 3.1: (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride / N,N-dimethyl-formamide / 5 h / 125 °C / Schlenk technique; Inert atmosphere View Scheme |
-
-
1358063-31-6
2-bromo-3-methoxy-6-vinylphenol

-
-
484-12-8
osthole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer; copper(II) acetate monohydrate / acetonitrile / 16 h / 85 °C 2: (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride / N,N-dimethyl-formamide / 5 h / 125 °C / Schlenk technique; Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran 1.) 15 min, 2.) 10 h; | 98% |
| With potassium hydroxide 1.) DMSO, 1 h, 2.) (Osthol : CH3I = 1:1) 12 h; Yield given. Multistep reaction; | |
| Stage #1: osthole; methyl iodide With potassium hydroxide In ethanol at 90℃; for 24h; Stage #2: With hydrogenchloride In ethanol; water pH=3 - 4; | |
| Stage #1: osthole; methyl iodide With ethanol; potassium hydroxide at 90℃; Stage #2: With hydrogenchloride In ethanol; water pH=3 - 4; |
-
-
484-12-8
osthole

-
-
69219-24-5
8-(3-hydroxy-3-methylbutyl)-7-methoxy-2H-chromen-2-one

| Conditions | Yield |
|---|---|
| Stage #1: osthole With mercury(II) diacetate; water In tetrahydrofuran; dichloromethane for 3h; Stage #2: With sodium tetrahydroborate In tetrahydrofuran; dichloromethane | 97% |
| With sulfuric acid; water In acetic acid at 0 - 5℃; for 1h; | 80% |
| With iron(III)-acetylacetonate; methyl 4-nitrobenzenesulfonate; phenylsilane; sodium hydrogencarbonate In methanol at 0 - 20℃; for 12h; Schlenk technique; Inert atmosphere; regioselective reaction; | 71% |
| With boron trifluoride diethyl etherate; water In dichloromethane Ambient temperature; | 52% |

| Conditions | Yield |
|---|---|
| Stage #1: osthole With sodium hydroxide In dimethyl sulfoxide at 25℃; for 0.5h; Stage #2: propargyl bromide In dimethyl sulfoxide at 20℃; for 2h; | 96% |

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane at -5℃; for 2h; | 93% |
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 0℃; | 88% |
| With Perbenzoic acid; chloroform | |
| Multi-step reaction with 2 steps 1: 180 mg / N-bromosuccinimide, DMSO, water / 1 h / Ambient temperature 2: 120 mg / sodium borohydride / dimethylsulfoxide / Ambient temperature View Scheme |
-
-
484-12-8
osthole

-
-
181303-71-9
8-isopentyl-7-methoxy-chroman-2-one

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In ethyl acetate at 20℃; under 760.051 Torr; for 16h; | 92% |
| With palladium 10% on activated carbon; hydrogen In ethyl acetate at 20℃; under 760.051 Torr; for 16h; | 92% |
| With palladium on activated charcoal; acetic acid Hydrogenation; |

| Conditions | Yield |
|---|---|
| With platinum(IV) oxide; hydrogen In ethanol at 20℃; | 91% |
| With palladium; ethyl acetate Hydrogenation; | |
| With acetic acid Hydrogenation; | |
| Multi-step reaction with 2 steps 1: 66 percent / H2 / Pd/C / CH2Cl2; ethanol 2: 72 percent / Pd/C / 1,3,5-trimethyl-benzene / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: palladium 10% on activated carbon; hydrogen / ethyl acetate / 16 h / 20 °C / 760.05 Torr 2: palladium 10% on activated carbon / 1,3,5-trimethyl-benzene / 16 h / 166 °C / Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| Stage #1: osthole With methanesulfonamide; AD-mix β In water; tert-butyl alcohol at 20℃; for 12h; Stage #2: With sodium sulfite In water; tert-butyl alcohol at 20℃; for 0.5h; | 90% |
| Multi-step reaction with 2 steps 1: 88 percent / m-chloroperbenzoic acid / CH2Cl2 / 0 °C 2: 73 percent / H2O, H2SO4 / tetrahydrofuran / Ambient temperature View Scheme |
-
-
484-12-8
osthole

| Conditions | Yield |
|---|---|
| Stage #1: N'-(3-methoxybenzylidene)-2-nitrobenzenesulfonohydrazide With sodium hydride In dichloromethane at 20℃; for 1h; Inert atmosphere; Sealed tube; Stage #2: osthole With silver trifluoromethanesulfonate In dichloromethane at 40℃; for 18h; Inert atmosphere; Sealed tube; | 85% |
-
-
484-12-8
osthole

-
-
1663-39-4
tert-Butyl acrylate

-
-
1299490-72-4
(E)-tert-butyl 4-(7-methoxy-2-oxo-2H-chromen-8-yl)but-2-enoate

| Conditions | Yield |
|---|---|
| With tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride; copper(l) iodide In diethyl ether at 35℃; for 24h; Inert atmosphere; diastereoselective reaction; | 81% |
-
-
484-12-8
osthole

-
-
75-03-6
ethyl iodide

-
-
73490-52-5
3-(2-ethoxy-4-methoxy-3-(3-methylbut-2-enyl)phenyl)acrylic acid

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran 1.) 15 min, 2.) heating; | 80% |
| With potassium hydroxide 1.) DMSO, 1 h, 2.) (Osthol : C2H5I = 1:1) 12 h; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| With L-Cysteine; sodium hydride In N,N-dimethyl-formamide for 3h; Reflux; | 77% |
| With aluminium trichloride; dimethylsulfide In dichloromethane at 30℃; for 24h; | 62% |
| Stage #1: osthole With aluminum (III) chloride; dimethylsulfide In dichloromethane at 0 - 30℃; Stage #2: With hydrogenchloride In dichloromethane; water | 62% |
| With aluminum (III) chloride; dimethylsulfide In dichloromethane at 0 - 30℃; for 24h; | 59% |

| Conditions | Yield |
|---|---|
| With aluminium trichloride; ethanethiol In dichloromethane at 30℃; for 24h; | 76% |
| With phosphorus; hydrogen bromide |
-
-
484-12-8
osthole

| Conditions | Yield |
|---|---|
| With Lawessons reagent In tetrahydrofuran for 24h; Reflux; | 76% |
| With Lawessons reagent In tetrahydrofuran at 66℃; for 24h; | 351 mg |
-
-
484-12-8
osthole

-
A
-
6619-22-3
8-isopentyl-7-methoxy-2H-chromen-2-one

-
B
-
181303-71-9
8-isopentyl-7-methoxy-chroman-2-one

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In ethanol; dichloromethane | A 21% B 66% |
-
-
484-12-8
osthole

| Conditions | Yield |
|---|---|
| Stage #1: osthole With water; sodium hydroxide for 8h; Reflux; Stage #2: With hydrogenchloride In water at 20℃; pH=2 - 3; | 65.7% |
-
-
484-12-8
osthole

-
-
73292-92-9
(2E)-4-(7-methoxy-2-oxo-2H-chromen-8-yl)-2-methylbut-2-enal

| Conditions | Yield |
|---|---|
| With selenium(IV) oxide; acetic acid for 3h; | 60% |

| Conditions | Yield |
|---|---|
| Stage #1: osthole With water; sodium hydroxide for 0.5h; Reflux; Stage #2: dimethyl sulfate With sodium hydroxide In water at 20℃; for 1.5h; Reflux; Stage #3: With hydrogenchloride In water at 20℃; pH=2 - 3; | 55.4% |
-
-
484-12-8
osthole

-
A
-
73292-92-9
(2E)-4-(7-methoxy-2-oxo-2H-chromen-8-yl)-2-methylbut-2-enal

-
B
-
73292-93-0
4'-hydroxylosthole

| Conditions | Yield |
|---|---|
| With selenium(IV) oxide In ethanol for 0.75h; Heating; | A 22% B 55% |

| Conditions | Yield |
|---|---|
| Stage #1: osthole With water; sodium hydroxide Reflux; Stage #2: dimethyl sulfate With sodium hydroxide In water at 20℃; for 3.5h; Reflux; Stage #3: With hydrogenchloride In water at 20℃; pH=2 - 3; | 52.2% |
-
-
484-12-8
osthole

| Conditions | Yield |
|---|---|
| With selenium(IV) oxide; acetic acid for 3h; | 50% |
| With selenium(IV) oxide In ethanol at 65℃; for 10h; Inert atmosphere; | |
| With selenium(IV) oxide In 1,4-dioxane at 60 - 80℃; for 1.5h; |
-
-
484-12-8
osthole

-
-
275818-95-6
sodium difluoromethanesulfinate

| Conditions | Yield |
|---|---|
| With eosin y In dimethyl sulfoxide at 20℃; for 24h; Schlenk technique; Irradiation; | 47% |

| Conditions | Yield |
|---|---|
| With tris[2-phenylpyridinato-C2,N]iridium(III); potassium carbonate In dimethyl sulfoxide at 20℃; for 12h; Schlenk technique; Irradiation; Inert atmosphere; | 43% |

| Conditions | Yield |
|---|---|
| With aluminium trichloride In dichloromethane at 30℃; for 12h; | A 20% B 40% |

-
-
484-12-8
osthole

| Conditions | Yield |
|---|---|
| With bromine In dichloromethane at 0℃; | 34% |
| With tetrachloromethane; bromine |

| Conditions | Yield |
|---|---|
| Stage #1: osthole With water; sodium hydroxide at 20℃; Reflux; Stage #2: dimethyl sulfate In water at 20℃; for 3h; Reflux; Stage #3: With hydrogenchloride In water at 20℃; pH=2 - 3; | 31.25% |
Osthole Chemical Properties
IUPAC Name: 7-Methoxy-8-(3-methylbut-2-enyl)chromen-2-one
Following is the structure of Osthole (CAS NO.484-12-8):
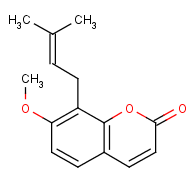
Empirical Formula: C15H16O3
Molecular Weight: 244.2857 g/mol
Index of Refraction: 1.556
Molar Refractivity: 69.79 cm3
Molar Volume: 216.8 cm3
Density: 1.126 g/cm3
Flash Point: 167.6 °C
Melting point: 83-84 °C
Surface Tension: 40.3 dyne/cm
Enthalpy of Vaporization: 64.71 kJ/mol
Boiling Point: 396.7 °C at 760 mmHg
Vapour Pressure of Osthole (CAS NO.484-12-8): 1.67E-06 mmHg at 25 °C
Product Categories of Osthole (CAS NO.484-12-8): Standard extract; Natural Plant Extract; Steroids
Water Solubility of Osthole (CAS NO.484-12-8): 12 mg/L at 30 °C
Canonical SMILES: CC(=CCC1=C(C=CC2=C1OC(=O)C=C2)OC)C
InChI: InChI=1S/C15H16O3/c1-10(2)4-7-12-13(17-3)8-5-11-6-9-14(16)18-15(11)12/h4-6,8-9H,7H2,1-3H3
InChIKey: MBRLOUHOWLUMFF-UHFFFAOYSA-N
Osthole Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LD | intravenous | > 40mg/kg (40mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 150, Pg. 425, 1964. | |
| guinea pig | LD | oral | > 1gm/kg (1000mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 150, Pg. 425, 1964. | |
| mouse | LD50 | intraperitoneal | 190mg/kg (190mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Indian Journal of Medical Research. Vol. 55, Pg. 241, 1967. |
| mouse | LD50 | subcutaneous | 16mg/kg (16mg/kg) | Pharmaceutical Chemistry Journal Vol. 11, Pg. 331, 1977. | |
| rabbit | LD | subcutaneous | > 70mg/kg (70mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 138, Pg. 400, 1962. | |
| rabbit | LDLo | intravenous | 25mg/kg (25mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: RESPIRATORY STIMULATION LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 138, Pg. 400, 1962. |
| rat | LD50 | intraperitoneal | 600mg/kg (600mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Indian Journal of Medical Research. Vol. 55, Pg. 241, 1967. |
| rat | LD50 | oral | 2905mg/kg (2905mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Indian Journal of Medical Research. Vol. 55, Pg. 241, 1967. |
Osthole Specification
Osthole , its cas register number is 484-12-8. It also can be called 7-Methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one ; 7-Methoxy-8-(3-methyl-2-butenyl)-coumarin ; 7-Methoxy-8-isopentenylcoumarin ; and 8-(3-Methyl-2-butenyl)herniarin . Adjuvants, immunologic; Calcium channel blockers; Cardiovascular Agents; Drug / Therapeutic Agent; Immunologic Factors and Membrane Transport Modulators.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View