-
Name
Phthalide
- EINECS 201-744-0
- CAS No. 87-41-2
- Article Data561
- CAS DataBase
- Density 1.265 g/cm3
- Solubility sparingly soluble in water
- Melting Point 71-74 °C(lit.)
- Formula C8H6O2
- Boiling Point 289.999 °C at 760 mmHg
- Molecular Weight 134.134
- Flash Point 124.844 °C
- Transport Information
- Appearance White to light beige crystalline powder
- Safety 26-36-24/25-37/39
- Risk Codes 36-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Phthalolactone;Phthalide(6CI,8CI);1-Phthalanone;2-Benzofuran-1(3H)-one;2-Hydroxymethylbenzoic acid g-lactone;3H-Isobenzofuran-1-one;NSC 1469;
- PSA 26.30000
- LogP 1.35700
Synthetic route

| Conditions | Yield |
|---|---|
| With (CH3)5C5*Ir(-OCH2C(C6H5)2NH-) In acetone at 20℃; for 4h; | 100% |
| With sodium bromate; hydrogen bromide In acetic acid at 35℃; for 5h; | 99% |
| With periodic acid; pyridinium chlorochromate In acetonitrile at 0 - 20℃; for 2h; | 99% |

| Conditions | Yield |
|---|---|
| With calcium oxide In benzene at 39.84℃; for 0.25h; Tishchenko reaction; | 100% |
| [La2(tBu2pz)6] In benzene-d6 at 21℃; Tishchenko reaction; | 100% |
| With [(ImDippN)Th{N(SiMe3)2}3] In benzene-d6 at 20℃; for 6h; | 100% |

| Conditions | Yield |
|---|---|
| With potassium carbonate; bis-triphenylphosphine-palladium(II) chloride In N,N-dimethyl-formamide at 60℃; under 760 Torr; for 72h; | 100% |
| With N-ethyl-N,N-diisopropylamine; C50H42O2P2Pd2; triphenylphosphine In toluene at 60℃; for 1.66667h; Conversion of starting material; | 100% |
| With triethylamine; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene; palladium dichloride In toluene at 80℃; under 760.051 Torr; for 4h; | 97% |

| Conditions | Yield |
|---|---|
| With oxygen at 340 - 360℃; Product distribution / selectivity; | A 0.01% B 100% |
| With oxygen at 340 - 360℃; | A 0.1% B 100% |
| With oxygen at 340 - 360℃; | A 0.03% B 100% |

| Conditions | Yield |
|---|---|
| With oxygen at 340 - 360℃; Product distribution / selectivity; | A 0.01% B 100% C 0.02% |
| With oxygen at 340 - 360℃; | A 0.03% B 100% C 0.05% |
| With oxygen at 340 - 360℃; | A 0.01% B 100% C 0.01% |


| Conditions | Yield |
|---|---|
| With oxygen at 340 - 360℃; | A 0.01% B 100% |
| With oxygen at 340 - 360℃; | A 0.01% B 100% |

| Conditions | Yield |
|---|---|
| With oxygen at 354 - 357℃; | A 0.2% B 100% C 0.15% |


| Conditions | Yield |
|---|---|
| With triethylsilane; iron(III)-acetylacetonate at 60℃; for 0.666667h; | 99% |
| With triethylsilane; iron(III)-acetylacetonate at 60℃; for 0.666667h; other catalysts: Co(acac)2, Ni(acac)2; same reaction of iso- and terephthaloyl chloride; | 99% |
| With tricyclohexylborane at 300℃; for 2h; | 11.5% |

| Conditions | Yield |
|---|---|
| With chromium(VI) oxide; periodic acid In acetonitrile at 20℃; for 0.25h; | 99% |
| With oxygen In dimethyl sulfoxide at 20℃; under 760.051 Torr; for 24h; Irradiation; | 99% |
| With tert.-butylhydroperoxide; 0.5C34H18N16(4-)*2H2O*2Cu(1+)*C3H7NO In water at 20℃; for 6h; Reagent/catalyst; Time; Sonication; | 97% |

| Conditions | Yield |
|---|---|
| With Li(1+)*C12H28AlO3(1-) In tetrahydrofuran; hexane at -78℃; for 1h; | 98% |
| With lithium borohydride In tetrahydrofuran for 0.25h; | 91% |
| With 4-butanolide; cyclohexane; hydrogen at 180℃; under 30003 Torr; for 4h; Catalytic behavior; Reagent/catalyst; Time; Autoclave; | 89.6% |
-
-
496-14-0
1,3-dihydroisobenzofuran

-
A
-
87-41-2
2-benzofuran-1(3H)-one

-
B
-
59868-79-0
1,3-dihydroisobenzofuran-1-ol

| Conditions | Yield |
|---|---|
| With cobalt(III) acetylacetonate; oxygen In ethyl acetate at 70℃; for 24h; | A 98% B 1% |

-
-
87-41-2
2-benzofuran-1(3H)-one

| Conditions | Yield |
|---|---|
| With methanesulfonic acid; acetic anhydride at 80℃; Temperature; Reagent/catalyst; | 98% |
| With sulfuric acid; acetic anhydride at 0 - 80℃; for 1h; |

| Conditions | Yield |
|---|---|
| With (dipyridylamine)Cp*IrOSO3; hydrogen In water at 130℃; under 3750.38 Torr; for 32h; | 97% |
| With formic acid; C20H29ClIrN4(1+)*Cl(1-) In water at 80℃; for 2h; chemoselective reaction; | 91% |
| With sodium tetrahydroborate; nickel(II) phthalocyanine In PEG-400 at 20℃; for 0.333333h; chemoselective reaction; | 87% |
| With iodine; phosphorous acid In 1,2-dichloro-ethane at 60℃; for 13h; Inert atmosphere; Schlenk technique; | 86% |
| Multi-step reaction with 2 steps 1: fluorobenzene / 0.5 h / 46 °C / Microwave irradiation 2: potassium carbonate / fluorobenzene / 0.17 h / 155 °C View Scheme |

| Conditions | Yield |
|---|---|
| Stage #1: terephthalaldehyde, With palladium 10% on activated carbon; sodium carbonate In isopropyl alcohol at 110℃; for 24h; Inert atmosphere; Stage #2: 2-iodobenzyl alcohol With triphenylphosphine In 1-methyl-pyrrolidin-2-one; isopropyl alcohol at 100℃; for 24h; Catalytic behavior; Solvent; Temperature; Inert atmosphere; | 97% |
| Stage #1: terephthalaldehyde, With palladium 10% on activated carbon; sodium carbonate In isopropyl alcohol at 120℃; for 24h; Inert atmosphere; Stage #2: 2-iodobenzyl alcohol With triphenylphosphine In 1-methyl-pyrrolidin-2-one at 100℃; for 24h; Inert atmosphere; | 85% |
-
-
51707-35-8
2-hydroxymethyl-benzoic acid hydrazide

-
-
87-41-2
2-benzofuran-1(3H)-one

| Conditions | Yield |
|---|---|
| With t-butyldimethylsiyl triflate; N,N-dimethyl-formamide at 20℃; for 20h; | 97% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-(bromomethyl)benzoic acid With 1,8-diazabicyclo[5.4.0]undec-7-ene In acetone for 0.166667h; Stage #2: With methyl iodide In acetone at 20℃; for 4h; | 96% |
| With cesium fluoride In acetonitrile for 10h; Heating; | 80% |
| With sodium hydroxide anschl. Ansaeuern; | |
| In gas at 380℃; Mechanism; Thermodynamic data; Ea(excit.); |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In tetrahydrofuran at 0 - 20℃; for 1.5h; | A n/a B 96% |

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; dicobalt octacarbonyl In sodium hydroxide; benzene at 65℃; for 2.5h; Irradiation; | 95% |
| With potassium carbonate; F-CH2Co(CO)4 In methanol at 25℃; | 84% |
| With di-μ-iodobis(tri-t-butylphosphino)dipalladium(l); triethylamine In toluene at 100℃; under 900.09 Torr; for 12h; | 82 %Chromat. |

| Conditions | Yield |
|---|---|
| With 5%-palladium/activated carbon; sodium fluoride In 1,4-dioxane at 150℃; for 24h; | 94% |

| Conditions | Yield |
|---|---|
| With 5%-palladium/activated carbon; sodium fluoride; 3-chloro-benzenecarboperoxoic acid In 1,4-dioxane at 150℃; for 24h; | 94% |
-
-
21483-54-5
4,7-dihyrophthalide

-
-
87-41-2
2-benzofuran-1(3H)-one

| Conditions | Yield |
|---|---|
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In toluene for 14h; Ambient temperature; | 92% |
-
-
15226-74-1, 61091-28-9, 61117-58-6
dicobalt octacarbonyl

-
-
64-17-5
ethanol

-
-
18982-54-2
o-bromobenzyl alcohol

-
-
87-41-2
2-benzofuran-1(3H)-one

| Conditions | Yield |
|---|---|
| With dmap; palladium diacetate; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In toluene at 90℃; for 0.5h; Microwave irradiation; | 92% |


| Conditions | Yield |
|---|---|
| With 1-methyl-1H-imidazole; [2,2]bipyridinyl; tetrakis(acetonitrile)copper(I) trifluoromethanesulfonate; 9-azabicyclo<3.3.1>nonane-N-oxyl In acetonitrile at 22℃; for 2h; | A 92% B 8% |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In methanol; tert-butyl alcohol Heating; | A 9% B 91% |
| With sodium tetrahydroborate In methanol; tert-butyl alcohol Heating; | A 8% B 91% |
| With diisobutylaluminium hydride In dichloromethane; toluene at -30 - 20℃; for 1h; Inert atmosphere; regioselective reaction; | A 68% B 10% |
| With diisobutylaluminium hydride In dichloromethane; toluene at -30 - 20℃; for 1h; Temperature; Inert atmosphere; regioselective reaction; | A 30% B 60% |

| Conditions | Yield |
|---|---|
| With oxygen In water at 80℃; for 12h; Reagent/catalyst; Sealed tube; | 90% |
| With copper(II) oxide; zinc In water at 250℃; for 2h; | 17% |
| With water |
-
-
85719-69-3
2-[(Diisopropoxy-methyl-silanyl)-methyl]-benzoic acid methyl ester

-
-
87-41-2
2-benzofuran-1(3H)-one

| Conditions | Yield |
|---|---|
| With peracetic acid; potassium fluoride In N,N-dimethyl-formamide for 18h; Ambient temperature; | 90% |

| Conditions | Yield |
|---|---|
| With dipotassium hydrogenphosphate; palladium diacetate at 140℃; for 36h; | 90% |
| With dipotassium hydrogenphosphate; palladium diacetate at 130℃; for 36h; | 87% |
| With palladium diacetate for 36h; Alkaline conditions; Heating; |

| Conditions | Yield |
|---|---|
| With tungsten(VI) oxide monohydrate; dihydrogen peroxide In water; tert-butyl alcohol at 80℃; for 24h; | 90% |

| Conditions | Yield |
|---|---|
| With triethylamine; nickel In tetrahydrofuran | 89% |
| With hydrogen In Pt on charcoal; 5,5-dimethyl-1,3-cyclohexadiene | |
| With sodium acetate; palladium In acetic acid |
-
-
643-79-8
o-phthalic dicarboxaldehyde

-
-
98-80-6
phenylboronic acid

-
A
-
87-41-2
2-benzofuran-1(3H)-one

-
B
-
5398-11-8
3-phenylphthalide

| Conditions | Yield |
|---|---|
| With potassium carbonate; 1,2-bis-(diphenylphosphino)ethane; cobalt(II) iodide In tetrahydrofuran at 80℃; for 12h; Inert atmosphere; | A 10% B 89% |
| With 1,10-Phenanthroline; potassium carbonate; cobalt(II) chloride In acetonitrile at 80℃; for 12h; Inert atmosphere; | A 65% B 36% |

-
-
87-41-2
2-benzofuran-1(3H)-one

-
-
75-16-1
methylmagnesium bromide

-
-
55549-01-4
α,α-dimethyl-o-xylene α,α'-diol

| Conditions | Yield |
|---|---|
| at -78 - 20℃; | 100% |
| In tetrahydrofuran; diethyl ether at 30℃; for 12.5h; | 88% |
| unter Wasserabgabe entsteht 1.1-Dimethyl-phthalan; |

| Conditions | Yield |
|---|---|
| In water at 220℃; under 114000 Torr; for 4h; | 100% |
| With ethanol at 220℃; | |
| In 5,5-dimethyl-1,3-cyclohexadiene |

| Conditions | Yield |
|---|---|
| With sodium aluminum tetrahydride In tetrahydrofuran at 0℃; for 0.25h; | 100% |
| With dimethyl zinc(II); sodium hydride In tetrahydrofuran at 0℃; for 6h; | 100% |
| With lithium aluminium tetrahydride In tetrahydrofuran at 25℃; under 760.051 Torr; for 16h; Inert atmosphere; | 99% |
-
-
87-41-2
2-benzofuran-1(3H)-one

-
-
100-53-8
phenylmethanethiol

-
-
1218-59-3
2-<(Benzylthio)methyl>benzoic acid

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide; mineral oil for 45h; Reflux; | 100% |
| With sodium hydride In N,N-dimethyl-formamide for 24h; Heating; | 99% |
| 58% | |
| With sodium ethanolate In ethanol |
-
-
67-56-1
methanol

-
-
87-41-2
2-benzofuran-1(3H)-one

-
-
34040-62-5
o-(chloromethyl)benzoic acid methyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 2-benzofuran-1(3H)-one With boron trifluoride diethyl etherate; N-benzyl-N,N,N-triethylammonium chloride In xylene at 100℃; Stage #2: With thionyl chloride In xylene at 100 - 132℃; for 3h; Stage #3: methanol at 50 - 60℃; for 2h; | 100% |
| Stage #1: 2-benzofuran-1(3H)-one With chloro(triphenyl)phosphonium chloride at 180℃; for 2h; Stage #2: methanol With pyridine for 1h; | |
| With pyridine; dichlorotriphenylphosphorane at 180℃; |
-
-
87-41-2
2-benzofuran-1(3H)-one

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; hexane | 100% |

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol for 2h; Reflux; | 100% |

| Conditions | Yield |
|---|---|
| In methanol for 36h; | 99.5% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-benzofuran-1(3H)-one With sodium hydroxide In water at 20℃; for 3h; Stage #2: p-methoxybenzyl chloride In N,N-dimethyl-formamide at 80℃; for 1h; | 99% |
| Stage #1: 2-benzofuran-1(3H)-one With water; sodium hydroxide In tetrahydrofuran at 20℃; for 3h; Stage #2: p-methoxybenzyl chloride In N,N-dimethyl-formamide at 80℃; for 1h; |
-
-
87-41-2
2-benzofuran-1(3H)-one

-
-
25015-63-8
4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane

| Conditions | Yield |
|---|---|
| With ToMMgMe at 24.84℃; for 1h; | 99% |
| With C12H36MgN2Si4 In neat (no solvent) at 20℃; for 0.25h; Catalytic behavior; Reagent/catalyst; Solvent; Inert atmosphere; Schlenk technique; | 99% |
| With C42H50Mg2N4 In neat (no solvent) at 25℃; for 1h; Glovebox; Schlenk technique; Inert atmosphere; | 99 %Spectr. |
-
-
87-41-2
2-benzofuran-1(3H)-one

-
-
5815-08-7
tert-Butoxybis(dimethylamino)methane

-
-
89968-08-1
3-isobenzofuran-1(3H)-one

| Conditions | Yield |
|---|---|
| at 135℃; for 0.5h; | 98% |
-
-
87-41-2
2-benzofuran-1(3H)-one

-
-
2052-49-5
tetra(n-butyl)ammonium hydroxide

-
-
93612-81-8
tetra-n-butylammonium 2-(hydroxymethyl)benzoate

| Conditions | Yield |
|---|---|
| for 0.5h; | 98% |
| With water for 1.5h; Heating; | 39 g |
| In water for 1.5h; Heating; |

| Conditions | Yield |
|---|---|
| Stage #1: 2-benzofuran-1(3H)-one With lithium diisopropyl amide In tetrahydrofuran at -78℃; Stage #2: butyraldehyde In tetrahydrofuran | 98% |
| With lithium diisopropyl amide 1.) THF, -78 deg C, 10 min, 2.) THF, -78 deg C, 1-2 h; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 40℃; for 3h; | 98% |
-
-
87-41-2
2-benzofuran-1(3H)-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water for 2h; Reflux; | 98% |

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In tetrachloromethane | 97% |
| With N-Bromosuccinimide In tetrachloromethane for 0.666667h; Bromination; Irradiation; | 94% |
| With N-Bromosuccinimide In tetrachloromethane for 0.666667h; Irradiation; | 94% |
-
-
87-41-2
2-benzofuran-1(3H)-one

-
-
59868-79-0
1,3-dihydroisobenzofuran-1-ol

| Conditions | Yield |
|---|---|
| With diisobutylaluminium hydride In hexane; dichloromethane at -78℃; for 1.5h; | 97% |
| With diisobutylaluminium hydride | 91% |
| With diisobutylaluminium hydride In dichloromethane at -78℃; | 70% |
Phthalide Consensus Reports
Phthalide Specification
The Phthalide, with the CAS registry number 87-41-2, is also known as 1(3H)-Isobenzofuranone. Its EINECS registry number is 201-744-0. This chemical's molecular formula is C8H6O2 and molecular weight is 134.13. What's more, its IUPAC name is called 3H-2-Benzofuran-1-one. Phthalide is a lactone that serves as the core chemical structure for a variety of more complex chemical compounds including dyes, fungicides and natural oils.
Physical properties about Phthalide are: (1)ACD/LogP: 1.029; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.03; (4)ACD/LogD (pH 7.4): 1.03; (5)ACD/BCF (pH 5.5): 3.56; (6)ACD/BCF (pH 7.4): 3.56; (7)ACD/KOC (pH 5.5): 86.43; (8)ACD/KOC (pH 7.4): 86.43; (9)#H bond acceptors: 2; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 0; (12)Polar Surface Area: 26.3 Å2; (13)Index of Refraction: 1.585; (14)Molar Refractivity: 35.551 cm3; (15)Molar Volume: 106.065 cm3; (16)Polarizability: 14.094×10-24 cm3; (17)Surface Tension: 47.17 dyne/cm; (18)Density: 1.265 g/cm3; (19)Flash Point: 124.844 °C; (20)Enthalpy of Vaporization: 52.934 kJ/mol; (21)Boiling Point: 289.999 °C at 760 mmHg; (22)Vapour Pressure: 0.0020 mmHg at 25 °C.
Preparation of Phthalide: this chemical can be prepared by 2-methyl-benzoic acid. This reaction needs reagents sodium bromate, sodium hydrogen sulfite and solvents ethyl acetate, H2O at temperature of 20 °C. The reaction time is 40 hours. The yield is 63 %.

Uses of Phthalide: (1)it is mainly used as intermediates in organic synthesis; (2) it is used to produce other chemicals. For example, it can react with methylmagnesium bromide to get 2-(a-hydroxy-isopropyl)-benzyl alcohol. The reaction occurs with diethyl ether at temperature of 20 °C. The yield is 100 %.
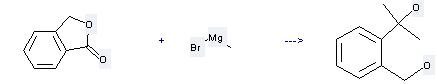
When you are dealing with this chemical, you should be very careful. This chemical is inflammation to the skin, eyes and respiratory system or other mucous membranes. Therefore, you should wear suitable protective clothing, gloves and eye/face protection. You must avoid contacting with skin and eyes. In case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1) SMILES: O=C1OCc2ccccc12
(2) InChI: InChI=1S/C8H6O2/c9-8-7-4-2-1-3-6(7)5-10-8/h1-4H,5H2
(3) InChIKey: WNZQDUSMALZDQF-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD | intraperitoneal | > 500mg/kg (500mg/kg) | National Academy of Sciences, National Research Council, Chemical-Biological Coordination Center, Review. Vol. 5, Pg. 25, 1953. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View