-
Name
Piperidine hydrochloride
- EINECS 228-033-8
- CAS No. 6091-44-7
- Article Data98
- CAS DataBase
- Density 1,12 g/cm3
- Solubility Soluble in water and ethanol
- Melting Point 245-249 °C
- Formula C5H11N.HCl
- Boiling Point 106.4 °C at 760 mmHg
- Molecular Weight 121.61
- Flash Point 4.4 °C
- Transport Information UN 2811 6.1/PG 3
- Appearance white fine crystalline powder
- Safety 36/37/39-45-26
- Risk Codes 36/37/38-23/24/25
-
Molecular Structure
-
Hazard Symbols
 T
T
- Synonyms Piperidine, hydrochloride (7CI,8CI,9CI);Piperidinium chloride;Piperidine, hydrochloride (1:1);Hexahydropyridine hydrochloride;NSC 27162;
- PSA 16.61000
- LogP -1.69310
Synthetic route
-
-
110-89-4
piperidine

-
A
-
123-56-8
Succinimide

-
B
-
25116-80-7
N,N'-thiodipiperidine

-
C
-
6091-44-7
piperidine hydrochloride

| Conditions | Yield |
|---|---|
| With N-chlorothiophthalimide In 1,2-dichloro-ethane at 20 - 25℃; for 2h; | A n/a B 77% C 100% |


| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride In ethanol for 2h; Reflux; | 100% |
| With tetrachloromethane; dicarbonylchloro(2,1,3-benzothiadiazole)rhodium(I) for 360h; Ambient temperature; also with other chlorinated solvents and rhodium complex catalysts; | 50% |
| Multi-step reaction with 2 steps 1: 18 percent / CHCl3 / 8 h / Heating 2: 0.4 g / HCl / diethyl ether View Scheme |

| Conditions | Yield |
|---|---|
| Stage #1: pyridine With palladium 10% on activated carbon; hydrogen; acetic acid at 24 - 28℃; for 15h; Stage #2: With hydrogenchloride In methanol; water Catalytic behavior; | 100% |
| Stage #1: pyridine With palladium on activated charcoal; hydrogen; acetic acid at 20℃; under 760.051 Torr; for 15h; Stage #2: With hydrogenchloride In methanol; water Time; | 100% |
| With hydrogenchloride; methanol; lithium triethylborohydride 1.) THF, RT. 0.5 h; Yield given. Multistep reaction; | |
| Stage #1: pyridine With ammonium formate; 10percent palladium on carbon In methanol at 20℃; for 16h; Stage #2: With hydrogenchloride In methanol Further stages.; | 95 % Spectr. |
| Stage #1: pyridine With hydrogen In 1,4-dioxane at 100℃; under 15001.5 Torr; for 3.33333h; Autoclave; Stage #2: With hydrogenchloride |

| Conditions | Yield |
|---|---|
| Stage #1: 2-chloropyridine-N-oxide With ammonium formate; 10percent palladium on carbon In methanol at 20℃; for 16h; Stage #2: With hydrogenchloride In methanol Further stages.; | 100% |
-
-
66203-96-1, 115871-51-7
N-(2-chloropropionyl)piperidine

-
-
6091-44-7
piperidine hydrochloride

| Conditions | Yield |
|---|---|
| With methanol at 50℃; for 0.5h; | 99% |

-
A
-
6091-44-7
piperidine hydrochloride

-
B
-
100461-60-7
2-(2'-nitrophenylsulphone)indene

| Conditions | Yield |
|---|---|
| With piperidine In tetrahydrofuran for 0.166667h; Ambient temperature; | A n/a B 99% |
-
-
75844-69-8
t-butyl piperidinecarboxylate

-
-
6091-44-7
piperidine hydrochloride

| Conditions | Yield |
|---|---|
| With sulfuric acid; sodium chloride In neat (no solvent) at 20℃; for 1h; Sealed tube; | 99% |

| Conditions | Yield |
|---|---|
| for 6h; | A n/a B 98% |


| Conditions | Yield |
|---|---|
| With 1,1,2-trichloroethane; 10% palladium on activated carbon; hydrogen In methanol under 760.051 Torr; for 1h; chemoselective reaction; | 98% |
| With dichloromethane; hydrogen; palladium on activated charcoal In methanol at 20℃; for 24h; atmospheric pressure; | 97% |
| With Na2K-SG(I) In tetrahydrofuran at 20℃; Inert atmosphere; | 82% |
| With hydrogenchloride; Vinyl chloroformate 1.) CH2Cl2, 0 deg C to room temperature;reflux, 4 h, 3.) MeOH, 50-60 deg C, 4.5 h; Yield given. Multistep reaction; |
-
-
2981-10-4
1-(cyclohex-1-en-1-yl)piperidine

-
-
32803-73-9
methyl 3-phenyl-3-chloro-2-ketopropionate

-
A
-
3626-62-8
2-phenyl-1-(1-piperidinyl)ethanone

-
C
-
6091-44-7
piperidine hydrochloride

| Conditions | Yield |
|---|---|
| In 1,4-dioxane for 2h; Reflux; | A 4% B 95% C 1% |

-
-
110-89-4
piperidine

-
-
75-09-2
dichloromethane

-
A
-
880-09-1
di(N-piperidinyl)methane

-
B
-
6091-44-7
piperidine hydrochloride

| Conditions | Yield |
|---|---|
| for 24h; Darkness; | A 91% B n/a |


| Conditions | Yield |
|---|---|
| With hydrogenchloride at 70℃; for 1h; acid degradation; | 88% |
-
-
14446-67-4
N-allylpiperidine

-
-
6091-44-7
piperidine hydrochloride

| Conditions | Yield |
|---|---|
| With Na2K-SG(I) In tetrahydrofuran at 20℃; Inert atmosphere; | 88% |

| Conditions | Yield |
|---|---|
| With perhydrodibenzo-18-crown-6 In diethylene glycol dimethyl ether for 3h; Ambient temperature; | 82% |
-
-
160727-95-7
methyl 3-chloro-3-(4-fluorophenyl)-2-oxopropanoate

-
-
67074-49-1
4-tert-butyl-1-(1-piperidinyl)cyclohexene


-
B
-
6091-44-7
piperidine hydrochloride

| Conditions | Yield |
|---|---|
| In 1,4-dioxane for 2h; Reflux; | A 82% B 11% |

-
-
110-89-4
piperidine

-
-
14783-10-9
[palladium(II)dichloride(1,10-phenanthroline)]

-
-
201230-82-2
carbon monoxide

-
-
301840-92-6
PdCl(CON(CH2)5)(1,10-phenanthroline)

-
B
-
6091-44-7
piperidine hydrochloride

| Conditions | Yield |
|---|---|
| In acetonitrile to suspn. PdCl2(phen) in CH3CN was added piperidine (1:2), suspn. was allowed to react under stirring with CO (0.1 MPa) at 313 K for 3 h; filtered off, washed with CH3CN/CH3OH (10:1), and dried in vacuo; elem.anal.; | A 80% B n/a |

-
-
110-89-4
piperidine

-
-
1258431-65-0
1-(thien-2-yl)-3,4,4-trichloro-3-buten-1-one

-
A
-
1258431-68-3
4,4-dichloro-3-piperidino-1-(thien-2-yl)-2-buten-1-one

-
B
-
6091-44-7
piperidine hydrochloride

| Conditions | Yield |
|---|---|
| In diethyl ether at 15℃; Reflux; | A 80% B n/a |


| Conditions | Yield |
|---|---|
| Stage #1: Glutaronitrile With C46H178O41Si42; titanium(IV)isopropoxide In toluene at 100℃; for 24h; Inert atmosphere; Stage #2: With hydrogenchloride; water In toluene at 20℃; for 4h; Inert atmosphere; chemoselective reaction; | 80% |
-
-
2981-10-4
1-(cyclohex-1-en-1-yl)piperidine

-
-
146736-30-3
methyl 3-(3-nitrophenyl)-3-chloro-2-oxopropionate

-
B
-
6091-44-7
piperidine hydrochloride

| Conditions | Yield |
|---|---|
| In 1,4-dioxane for 2h; Reflux; | A 80% B 15% |


| Conditions | Yield |
|---|---|
| In dichloromethane to soln. of azide was added soln. of BCl3 at -78°C; mixt. was allowed to warm up to room temp. overnight, MeOH added and solvents removed; characterized as benzoyl deriv.; | 79% |
-
-
110-89-4
piperidine

-
-
1258431-66-1
1-(4-hydroxyphenyl)-3,4,4-trichloro-3-buten-1-one

-
A
-
1258431-71-8
4,4-dichloro-3-piperidino-1-(4-hydroxyphenyl)-2-buten-1-one

-
B
-
6091-44-7
piperidine hydrochloride

| Conditions | Yield |
|---|---|
| In diethyl ether at 15℃; Reflux; | A 79% B n/a |

-
-
32803-73-9
methyl 3-phenyl-3-chloro-2-ketopropionate

-
-
67074-49-1
4-tert-butyl-1-(1-piperidinyl)cyclohexene

-
B
-
6091-44-7
piperidine hydrochloride

| Conditions | Yield |
|---|---|
| In 1,4-dioxane for 2h; Reflux; | A 79% B 15% |

-
-
110-89-4
piperidine

-
-
14871-92-2
dichloro(2,2'-bipyridine)palladium(II)

-
-
201230-82-2
carbon monoxide

-
-
301840-91-5
PdCl(CON(CH2)5)(2,2'-dipyridine)

-
B
-
6091-44-7
piperidine hydrochloride

| Conditions | Yield |
|---|---|
| In acetonitrile to suspn. PdCl2(dipy) in CH3CN was added piperidine (1:2), suspn. was allowed to react under stirring with CO (0.1 MPa) at room temp. for 3 h; filtered off, washed with CH3CN/CH3OH (10:1), and dried in vacuo; elem.anal.; | A 78% B n/a |


| Conditions | Yield |
|---|---|
| Stage #1: piperidine; furfural In diethyl ether at 20℃; for 1.5h; Stage #2: With acetyl chloride In diethyl ether at 20℃; for 0.333333h; Further stages.; | A 7% B 77% |


| Conditions | Yield |
|---|---|
| With perhydrodibenzo-18-crown-6 In diethylene glycol dimethyl ether for 3h; Ambient temperature; | 76% |
-
-
2981-10-4
1-(cyclohex-1-en-1-yl)piperidine

-
-
1108172-76-4
3-(3-bromo-phenyl)-3-chloro-2-oxo-propionic acid methyl ester

-
B
-
6091-44-7
piperidine hydrochloride

| Conditions | Yield |
|---|---|
| In 1,4-dioxane for 2h; Reflux; | A 76% B 18% |

-
-
2981-10-4
1-(cyclohex-1-en-1-yl)piperidine

-
-
146736-31-4
3-Chloro-3-(4-nitro-phenyl)-2-oxo-propionic acid methyl ester

-
B
-
6091-44-7
piperidine hydrochloride

-
C
-
105072-35-3
2-(4-nitrophenyl)-1-(piperidin-1-yl)ethan-1-one

| Conditions | Yield |
|---|---|
| In 1,4-dioxane for 2h; Reflux; | A 76% B 14% C 6% |

-
-
2981-10-4
1-(cyclohex-1-en-1-yl)piperidine

-
-
53173-90-3
methyl 3-chloro-3-(4-chlorophenyl)-2-oxopropanoate

-
B
-
6091-44-7
piperidine hydrochloride

| Conditions | Yield |
|---|---|
| In 1,4-dioxane for 2h; Reflux; | A 75% B 12% |

-
-
110-89-4
piperidine

-
-
14871-92-2
dichloro(2,2'-bipyridine)palladium(II)

-
-
201230-82-2
carbon monoxide

-
-
301840-95-9
Pd(CON(CH2)5)2(2,2'-dipyridine)

-
B
-
6091-44-7
piperidine hydrochloride

| Conditions | Yield |
|---|---|
| In acetonitrile to suspn. PdCl2(dipy) in MeCN was added piperidine (1:10), mixt. was reacted with CO (0.1 MPa) at room temp. for 2 h; mixt. was concd. and filtered; elem. anal.; | A 72% B n/a |

-
-
130004-33-0, 145926-28-9, 376354-47-1
(2,2'-bis(diphenylphosphino)-1,1'-binaphathalene)chloro(p-cymene)ruthenium chloride

-
-
6091-44-7
piperidine hydrochloride

| Conditions | Yield |
|---|---|
| In tetrahydrofuran (Ar); heating a soln. of ruthenium complex with ammonium salt in THF for 6 h; evapn., washing; | 100% |

-
-
6091-44-7
piperidine hydrochloride

| Conditions | Yield |
|---|---|
| With triethylamine In methanol at 20℃; | 99% |

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In ethanol at 30℃; for 22h; Mannich Aminomethylation; | 98% |

-
-
6091-44-7
piperidine hydrochloride

-
-
98897-40-6
sodium N,N-dimethylcarbamoselenothioate

-
-
98897-41-7
Piperidinium N,N-dimethylthioselenocarbamate

| Conditions | Yield |
|---|---|
| In methanol | 97% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 90℃; for 8h; | 96% |

-
-
50-00-0
formaldehyd

-
-
6091-44-7
piperidine hydrochloride

-
-
14823-31-5
4-Oxo-1-phenyl-4,5,6,7-tetrahydrobenopyrazole

| Conditions | Yield |
|---|---|
| With hydrogenchloride In isopropyl alcohol for 60h; Heating; | 95% |

-
-
646-06-0
1,3-DIOXOLANE

-
-
27465-51-6
1-(4-ethylphenyl)-1-propanone

-
-
6091-44-7
piperidine hydrochloride

-
-
56839-43-1
eperisone hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 90℃; for 6.5h; | 95% |

-
-
646-06-0
1,3-DIOXOLANE

-
-
6091-44-7
piperidine hydrochloride

-
-
93-55-0
1-phenyl-propan-1-one

-
-
6281-80-7
(+/-)-2-methyl-1-phenyl-3-piperidino-propan-1-one; hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 90℃; for 8h; Product distribution; other ketones, var. solvents and acids; | 95% |
| With hydrogenchloride at 90℃; for 8h; | 95% |

-
-
110-89-4
piperidine

-
-
5264-35-7
2-methoxy-1-pyrroline

-
-
6091-44-7
piperidine hydrochloride

-
-
1196-64-1
1-(3,4-dihydro-2H-pyrrol-5-yl)piperidine

| Conditions | Yield |
|---|---|
| at 70℃; for 24h; | 95% |

-
-
6091-44-7
piperidine hydrochloride

-
-
59340-27-1
1,3,5-trimethyl-1H-pyrazole-4-sulfonic acid chloride

| Conditions | Yield |
|---|---|
| Stage #1: piperidine hydrochloride With magnesium sulfate In dichloromethane at 20℃; for 0.25h; Stage #2: 1,3,5-trimethyl-1H-pyrazole-4-sulfonic acid chloride With triethylsilane In dichloromethane at 20℃; | 95% |
-
-
6091-44-7
piperidine hydrochloride

-
-
94696-08-9
sodium salt of 3-(1-adamantyl)-1-hydroxy-1-propen-3-one

| Conditions | Yield |
|---|---|
| In ethanol for 12h; Substitution; Heating; | 91% |
| In ethanol for 12h; Heating; | 91% |
-
-
75-75-2
methanesulfonic acid

-
-
5337-93-9
4'-methylpropiophenone

-
-
6091-44-7
piperidine hydrochloride

-
-
728-88-1, 67499-64-3
tolperisone

| Conditions | Yield |
|---|---|
| With 1,3-DIOXOLANE at 80℃; for 24h; Sealed tube; | 91% |

-
-
54750-12-8
(2R,4S,5R)-2-chloro-3,4-dimethyl-5-phenyl-1,3,2-oxazaphospholidin-2-one

-
-
6091-44-7
piperidine hydrochloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane Substitution; | 90% |
-
-
1445-45-0
Trimethyl orthoacetate

-
-
6091-44-7
piperidine hydrochloride

-
-
618-42-8
1-piperidin-1-yl-ethanone

| Conditions | Yield |
|---|---|
| In methanol at 135℃; for 0.25h; Microwave irradiation; | 90% |

| Conditions | Yield |
|---|---|
| With sodium carbonate In toluene at 60℃; Autoclave; | 90% |
-
-
13532-18-8
methyl 3-(methylthio)propionate

-
-
6091-44-7
piperidine hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: piperidine hydrochloride With diisobutylaluminium hydride In tetrahydrofuran; toluene Stage #2: methyl 3-(methylthio)propionate In tetrahydrofuran at 45℃; for 2h; | 89% |
-
-
6091-44-7
piperidine hydrochloride

-
-
2564-02-5
N-(4-bromophenyl)-2-chloroacetamide

-
-
58479-86-0
N-(4-bromophenyl)-2-(1-piperidyl)acetamide

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 80℃; for 6h; | 89% |
Piperidine hydrochloride Consensus Reports
Reported in EPA TSCA Inventory.
Piperidine hydrochloride Specification
The Piperidine hydrochloride, with the CAS registry number 6091-44-7, is also known as Piperidine, hydrochloride (1:1). It belongs to the product categories of Piperidine; Building Blocks; Heterocyclic Building Blocks; Piperidines. Its EINECS number is 228-033-8. This chemical's molecular formula is C5H11N.HCl and molecular weight is 121.63. What's more, its systematic name is Piperidinium chloride. Its classification code is Drug / Therapeutic Agent. It is stable at common pressure and temperature, and it should be sealed and stored in a cool and dry place. Moreover, it should be protected from light. This chemical is used in organic synthesis.
Physical properties of Piperidine hydrochloride are: (1)ACD/LogP: 0.932; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -2.17; (4)ACD/LogD (pH 7.4): -2.10; (5)ACD/BCF (pH 5.5): 1.00; (6)ACD/BCF (pH 7.4): 1.00; (7)ACD/KOC (pH 5.5): 1.00; (8)ACD/KOC (pH 7.4): 1.00; (9)#H bond acceptors: 1; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 0; (12)Flash Point: 4.4 °C; (13)Enthalpy of Vaporization: 34.53 kJ/mol; (14)Boiling Point: 106.4 °C at 760 mmHg; (15)Vapour Pressure: 28.3 mmHg at 25°C.
Preparation: this chemical can be prepared by N-(2-chloropropionyl)piperidine at the temperature of 50 °C. This reaction will need reagent methanol with the reaction time of 30 min. The yield is about 99%.

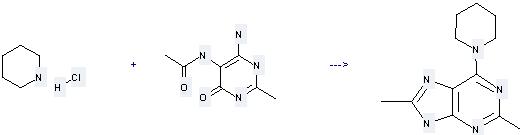
Uses of Piperidine hydrochloride: it can be used to produce 2,8-Dimethyl-6-(1-piperidyl)-9H-purin at the temperature of 200 °C. It will need reagents P2O5, NEt3·HCl with the reaction time of 3 hours. The yield is about 76%.
When you are using this chemical, please be cautious about it as the following:
This chemical is toxic by inhalation, in contact with skin and if swallowed. It is irritating to eyes, respiratory system and skin. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need to wear suitable protective clothing, gloves and eye/face protection. In case of accident or if you feel unwell, you must seek medical advice immediately (show the label where possible).
You can still convert the following datas into molecular structure:
(1)SMILES: [Cl-].[NH2+]1CCCCC1
(2)Std. InChI: InChI=1S/C5H11N.ClH/c1-2-4-6-5-3-1;/h6H,1-5H2;1H
(3)Std. InChIKey: VEIWYFRREFUNRC-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| frog | LDLo | subcutaneous | 1gm/kg (1000mg/kg) | LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION CARDIAC: PULSE RATE AUTONOMIC NERVOUS SYSTEM: OTHER (DIRECT) PARASYMPATHOMIMETIC | Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 50, Pg. 199, 1903. |
| mouse | LD50 | intraperitoneal | 330mg/kg (330mg/kg) | BEHAVIORAL: ATAXIA LUNGS, THORAX, OR RESPIRATION: CYANOSIS BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Japanese Journal of Pharmacology. Vol. 17, Pg. 475, 1967. |
| mouse | LD50 | intravenous | 160mg/kg (160mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 112, Pg. 36, 1957. | |
| mouse | LDLo | subcutaneous | 657mg/kg (657mg/kg) | Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 50, Pg. 199, 1903. | |
| mouse | LDLo | subcutaneous | 657mg/kg (657mg/kg) | LUNGS, THORAX, OR RESPIRATION: CYANOSIS AUTONOMIC NERVOUS SYSTEM: OTHER (DIRECT) PARASYMPATHOMIMETIC | Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 50, Pg. 199, 1903. |
| rat | LD50 | oral | 133mg/kg (133mg/kg) | "Psychotropic Drugs and Related Compounds," 2nd ed., Usdin, E., and D.H. Efron, Washington, DC, 1972Vol. -, Pg. 289, 1972. |
Related Products
- Piperidine
- Piperidine hydrochloride
- Piperidine pentamethylenedithiocarbamate
- Piperidine, 1-((methylsulfinyl)-1-pyrrolidinylphosphinyl)-, (R*,R*)-
- Piperidine, 1-(3-(3,5-bis(trifluoromethyl)phenyl)-2-propynyl)-4-(1,1-dimethylethyl)-, hydrochloride
- Piperidine, 1-(iodomethyl)-
- Piperidine, 1-acetyl-4-(2-hydroxy-4-methoxybenzoyl)-
- Piperidine, 1-acetyl-4-[3-(trifluoromethyl)benzoyl]-
- Piperidine, 1-ethyl-,1-oxide
- Piperidine, 3-ethoxy-
- 609-15-4
- 6091-64-1
- 60919-46-2
- 609-19-8
- 609-20-1
- 60920-20-9
- 60920-72-1
- 60920-82-3
- 609-21-2
- 6092-18-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View