-
Name
Repaglinide
- EINECS 629-921-1
- CAS No. 135062-02-1
- Article Data16
- CAS DataBase
- Density 1.137 g/cm3
- Solubility DMSO: 34 mg/mL
- Melting Point 129-130.2 °C
- Formula C27H36N2O4
- Boiling Point 672.9 °C at 760 mmHg
- Molecular Weight 452.594
- Flash Point 360.8 °C
- Transport Information
- Appearance white to off-white solid
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms (S)-(+)-2-Ethoxy-4-[N-[1-(2-piperidinophenyl)-3-methyl-1-butyl]aminocarbonylmethyl]benzoic acid;Novonorm;Prandin;
- PSA 78.87000
- LogP 5.67580
Synthetic route
-
-
147770-06-7
(S)-(+)-ethyl 2-ethoxy-4-<2-<<3-methyl-1-<2-(1-piperidinyl)phenyl>butyl>amino>-2-oxoethyl>-benzoate

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| With ethanol; water; sodium hydroxide Reflux; | 96% |
| With sodium hydroxide In ethanol at 60℃; for 4h; | 92% |
| With methanol; sodium hydroxide for 3h; Reflux; | 87.6% |

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| With potassium hydroxide In acetonitrile for 6h; Reflux; | 89.9% |

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| With ethanol; sodium hydroxide for 5h; Reflux; | 85.1% |
-
-
110-89-4
piperidine

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: 64.5 g / 6 h / 220 - 225 °C 2.1: Mg; I2 / tetrahydrofuran / 1 h / 60 - 65 °C / Heating 2.2: toluene; tetrahydrofuran / 14 h / 98 - 102 °C 3.1: 68.9 g / NaBH4 / methanol / 10 - 15 °C 4.1: N-acetyl-L-glutamic acid / acetone 5.1: 80 percent / N,N-dicyclohexylcarbodiimide / CH2Cl2 / 20 °C 6.1: aq. NaOH / propan-2-ol / 60 - 65 °C View Scheme |
-
-
72752-52-4
2-(piperidin-1-yl)benzonitrile

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: Mg; I2 / tetrahydrofuran / 1 h / 60 - 65 °C / Heating 1.2: toluene; tetrahydrofuran / 14 h / 98 - 102 °C 2.1: 68.9 g / NaBH4 / methanol / 10 - 15 °C 3.1: N-acetyl-L-glutamic acid / acetone 4.1: 80 percent / N,N-dicyclohexylcarbodiimide / CH2Cl2 / 20 °C 5.1: aq. NaOH / propan-2-ol / 60 - 65 °C View Scheme |
-
-
873-32-5
2-Chlorobenzonitrile

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: 64.5 g / 6 h / 220 - 225 °C 2.1: Mg; I2 / tetrahydrofuran / 1 h / 60 - 65 °C / Heating 2.2: toluene; tetrahydrofuran / 14 h / 98 - 102 °C 3.1: 68.9 g / NaBH4 / methanol / 10 - 15 °C 4.1: N-acetyl-L-glutamic acid / acetone 5.1: 80 percent / N,N-dicyclohexylcarbodiimide / CH2Cl2 / 20 °C 6.1: aq. NaOH / propan-2-ol / 60 - 65 °C View Scheme |
-
-
147769-93-5
(1S)-3-methyl-1-(2-piperidin-1-ylphenyl)butan-1-amine

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 80 percent / N,N-dicyclohexylcarbodiimide / CH2Cl2 / 20 °C 2: aq. NaOH / propan-2-ol / 60 - 65 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 29 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 2: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: 4-methyl-morpholine; pivaloyl chloride / dichloromethane / 1 h / 0 - 5 °C 1.2: 15 h / 0 - 20 °C 2.1: sodium hydroxide / ethanol / 1 h / 20 °C / Reflux View Scheme | |
| Multi-step reaction with 2 steps 1: tetramethylorthosilicate / toluene / 10 h / Inert atmosphere; Reflux 2: sodium hydroxide; ethanol / 5 h / Reflux View Scheme | |
| Multi-step reaction with 2 steps 1: tetramethylorthosilicate / toluene / 11 h / Inert atmosphere; Reflux 2: sodium hydroxide; methanol / 3 h / Reflux View Scheme |
-
-
108157-52-4
(S)-1-(2-piperidino-phenyl)-3-methyl-1-butylamine

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: N-acetyl-L-glutamic acid / acetone 2: 80 percent / N,N-dicyclohexylcarbodiimide / CH2Cl2 / 20 °C 3: aq. NaOH / propan-2-ol / 60 - 65 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 85 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 3: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme | |
| Multi-step reaction with 3 steps 2: 29 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 3: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme |
-
-
147769-96-8
3-methyl-1-[2-(piperidin-1-yl)phenyl]butan-1-imine

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 68.9 g / NaBH4 / methanol / 10 - 15 °C 2: N-acetyl-L-glutamic acid / acetone 3: 80 percent / N,N-dicyclohexylcarbodiimide / CH2Cl2 / 20 °C 4: aq. NaOH / propan-2-ol / 60 - 65 °C View Scheme | |
| Multi-step reaction with 5 steps 1: 34 percent / toluene / 15 h / Ambient temperature 2: H2, NaOH, Et3N, Ti(OiPr)4 / Ru(OAc)2<(S)-BINAP> / methanol; CH2Cl2 / 170 h / 30 °C / 75006 Torr 3: aq. HCl / 5.5 h / Heating 4: 29 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 5: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme | |
| Multi-step reaction with 6 steps 1: 4N aq. HCl / toluene; tetrahydrofuran / 20 °C 2: 63 percent / Et3N, TiCl4 / toluene / Ambient temperature 3: 76 percent / H2, Ti(O-iPr)4 / Raney-Ni / ethanol / 72 h / 50 °C / 150012 Torr 4: H2, aq. HCl / 10 percent Pd/C / H2O / 5 h / 50 °C / 3750.3 Torr 5: 29 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 6: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 84 percent / N-formyl-piperidine / 64 h / 160 - 170 °C 2: tetrahydrofuran; toluene / 3 h / Heating 3: 34 percent / toluene / 15 h / Ambient temperature 4: H2, NaOH, Et3N, Ti(OiPr)4 / Ru(OAc)2<(S)-BINAP> / methanol; CH2Cl2 / 170 h / 30 °C / 75006 Torr 5: aq. HCl / 5.5 h / Heating 6: 29 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 7: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme | |
| Multi-step reaction with 8 steps 1: 84 percent / N-formyl-piperidine / 64 h / 160 - 170 °C 2: tetrahydrofuran; toluene / 3 h / Heating 3: 4N aq. HCl / toluene; tetrahydrofuran / 20 °C 4: 63 percent / Et3N, TiCl4 / toluene / Ambient temperature 5: 76 percent / H2, Ti(O-iPr)4 / Raney-Ni / ethanol / 72 h / 50 °C / 150012 Torr 6: H2, aq. HCl / 10 percent Pd/C / H2O / 5 h / 50 °C / 3750.3 Torr 7: 29 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 8: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme | |
| Multi-step reaction with 8 steps 1: 84 percent / N-formyl-piperidine / 64 h / 160 - 170 °C 2: tetrahydrofuran; toluene / 3 h / Heating 3: 4N aq. HCl / toluene; tetrahydrofuran / 20 °C 4: Et3N, TiCl4 / toluene / Ambient temperature 5: 66 percent / H2, Ti(O-iPr)4 / Raney-Ni / ethanol / 160 h / 160 °C / 150012 Torr 6: H2, aq. HCl / 10 percent Pd/C / 10 h / 50 °C / 3750.3 Torr 7: 29 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 8: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme |
-
-
72752-52-4
2-(piperidin-1-yl)benzonitrile

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: tetrahydrofuran; toluene / 3 h / Heating 2: 34 percent / toluene / 15 h / Ambient temperature 3: H2, NaOH, Et3N, Ti(OiPr)4 / Ru(OAc)2<(S)-BINAP> / methanol; CH2Cl2 / 170 h / 30 °C / 75006 Torr 4: aq. HCl / 5.5 h / Heating 5: 29 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 6: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme | |
| Multi-step reaction with 7 steps 1: tetrahydrofuran; toluene / 3 h / Heating 2: 4N aq. HCl / toluene; tetrahydrofuran / 20 °C 3: 63 percent / Et3N, TiCl4 / toluene / Ambient temperature 4: 76 percent / H2, Ti(O-iPr)4 / Raney-Ni / ethanol / 72 h / 50 °C / 150012 Torr 5: H2, aq. HCl / 10 percent Pd/C / H2O / 5 h / 50 °C / 3750.3 Torr 6: 29 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 7: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme | |
| Multi-step reaction with 7 steps 1: tetrahydrofuran; toluene / 3 h / Heating 2: 4N aq. HCl / toluene; tetrahydrofuran / 20 °C 3: Et3N, TiCl4 / toluene / Ambient temperature 4: 66 percent / H2, Ti(O-iPr)4 / Raney-Ni / ethanol / 160 h / 160 °C / 150012 Torr 5: H2, aq. HCl / 10 percent Pd/C / 10 h / 50 °C / 3750.3 Torr 6: 29 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 7: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme |
-
-
873-32-5
2-Chlorobenzonitrile

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 84 percent / N-formyl-piperidine / 64 h / 160 - 170 °C 2: tetrahydrofuran; toluene / 3 h / Heating 3: 34 percent / toluene / 15 h / Ambient temperature 4: H2, NaOH, Et3N, Ti(OiPr)4 / Ru(OAc)2<(S)-BINAP> / methanol; CH2Cl2 / 170 h / 30 °C / 75006 Torr 5: aq. HCl / 5.5 h / Heating 6: 29 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 7: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme | |
| Multi-step reaction with 8 steps 1: 84 percent / N-formyl-piperidine / 64 h / 160 - 170 °C 2: tetrahydrofuran; toluene / 3 h / Heating 3: 4N aq. HCl / toluene; tetrahydrofuran / 20 °C 4: 63 percent / Et3N, TiCl4 / toluene / Ambient temperature 5: 76 percent / H2, Ti(O-iPr)4 / Raney-Ni / ethanol / 72 h / 50 °C / 150012 Torr 6: H2, aq. HCl / 10 percent Pd/C / H2O / 5 h / 50 °C / 3750.3 Torr 7: 29 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 8: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme | |
| Multi-step reaction with 8 steps 1: 84 percent / N-formyl-piperidine / 64 h / 160 - 170 °C 2: tetrahydrofuran; toluene / 3 h / Heating 3: 4N aq. HCl / toluene; tetrahydrofuran / 20 °C 4: Et3N, TiCl4 / toluene / Ambient temperature 5: 66 percent / H2, Ti(O-iPr)4 / Raney-Ni / ethanol / 160 h / 160 °C / 150012 Torr 6: H2, aq. HCl / 10 percent Pd/C / 10 h / 50 °C / 3750.3 Torr 7: 29 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 8: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme |
-
-
50-85-1
2-hydroxy-p-toluic acid

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: K2CO3 / diethyl ether / 30 h / 150 °C 2: N-bromosuccinimide, 2,2'-azo-bis-(isobutyronitril) / CCl4 3: 97 percent / aq. N-benzyl-tri-n-butylammonium-chloride / CH2Cl2 / 43 h / 20 °C 4: HCl / Heating 5: 59 percent / NaOH / ethanol / 1.5 h / 23 - 25 °C 6: 29 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 7: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme | |
| Multi-step reaction with 8 steps 1: K2CO3 / diethyl ether / 30 h / 150 °C 2: N-bromosuccinimide, 2,2'-azo-bis-(isobutyronitril) / CCl4 3: 97 percent / aq. N-benzyl-tri-n-butylammonium-chloride / CH2Cl2 / 43 h / 20 °C 4: HCl / Heating 5: 59 percent / NaOH / ethanol / 1.5 h / 23 - 25 °C 6: 85 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 8: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme | |
| Multi-step reaction with 7 steps 1: K2CO3 / diethyl ether / 30 h / 150 °C 2: N-bromosuccinimide, 2,2'-azo-bis-(isobutyronitril) / CCl4 3: 97 percent / aq. N-benzyl-tri-n-butylammonium-chloride / CH2Cl2 / 43 h / 20 °C 4: HCl / Heating 5: 59 percent / NaOH / ethanol / 1.5 h / 23 - 25 °C 6: 59 percent / N,N'-dicyclohexyl-carbodiimide / toluene / 2 h / Ambient temperature 7: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme |
-
-
88709-17-5
ethyl 2-ethoxy-4-methyl-benzoate

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: N-bromosuccinimide, 2,2'-azo-bis-(isobutyronitril) / CCl4 2: 97 percent / aq. N-benzyl-tri-n-butylammonium-chloride / CH2Cl2 / 43 h / 20 °C 3: HCl / Heating 4: 59 percent / NaOH / ethanol / 1.5 h / 23 - 25 °C 5: 29 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 6: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme | |
| Multi-step reaction with 7 steps 1: N-bromosuccinimide, 2,2'-azo-bis-(isobutyronitril) / CCl4 2: 97 percent / aq. N-benzyl-tri-n-butylammonium-chloride / CH2Cl2 / 43 h / 20 °C 3: HCl / Heating 4: 59 percent / NaOH / ethanol / 1.5 h / 23 - 25 °C 5: 85 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 7: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme | |
| Multi-step reaction with 6 steps 1: N-bromosuccinimide, 2,2'-azo-bis-(isobutyronitril) / CCl4 2: 97 percent / aq. N-benzyl-tri-n-butylammonium-chloride / CH2Cl2 / 43 h / 20 °C 3: HCl / Heating 4: 59 percent / NaOH / ethanol / 1.5 h / 23 - 25 °C 5: 59 percent / N,N'-dicyclohexyl-carbodiimide / toluene / 2 h / Ambient temperature 6: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme |
-
-
34595-26-1
2-(piperidin-1-yl)benzaldehyde

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: Ambient temperature 2: Ti(OiPr)4 / tetrahydrofuran; toluene / 60 h / 100 °C 3: H2, aq. HCl / 10 percent Pd/C / 5 h / 50 °C / 3750.3 Torr 4: 29 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 5: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: titanium(IV) tetraethanolate / tetrahydrofuran / 20 - 65 °C 2.1: magnesium / tetrahydrofuran; dichloromethane / 1 h / -50 - 20 °C 3.1: hydrogenchloride / water / 20 °C 4.1: 4-methyl-morpholine; pivaloyl chloride / dichloromethane / 1 h / 0 - 5 °C 4.2: 15 h / 0 - 20 °C 5.1: sodium hydroxide / ethanol / 1 h / 20 °C / Reflux View Scheme | |
| Multi-step reaction with 5 steps 1.1: titanium(IV) tetraethanolate / tetrahydrofuran / 20 - 65 °C 2.1: magnesium / tetrahydrofuran; dichloromethane / 1 h / -50 - 20 °C 3.1: hydrogenchloride / water / 20 °C 4.1: 4-methyl-morpholine; pivaloyl chloride / dichloromethane / 1 h / 0 - 5 °C 4.2: 15 h / 0 - 20 °C 5.1: sodium hydroxide / ethanol / 1 h / 20 °C / Reflux View Scheme |
-
-
110017-07-7
ethyl 4-bromomethyl-2-ethoxy-benzoate

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 97 percent / aq. N-benzyl-tri-n-butylammonium-chloride / CH2Cl2 / 43 h / 20 °C 2: HCl / Heating 3: 59 percent / NaOH / ethanol / 1.5 h / 23 - 25 °C 4: 29 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 5: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme | |
| Multi-step reaction with 6 steps 1: 97 percent / aq. N-benzyl-tri-n-butylammonium-chloride / CH2Cl2 / 43 h / 20 °C 2: HCl / Heating 3: 59 percent / NaOH / ethanol / 1.5 h / 23 - 25 °C 4: 85 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 6: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme | |
| Multi-step reaction with 5 steps 1: 97 percent / aq. N-benzyl-tri-n-butylammonium-chloride / CH2Cl2 / 43 h / 20 °C 2: HCl / Heating 3: 59 percent / NaOH / ethanol / 1.5 h / 23 - 25 °C 4: 59 percent / N,N'-dicyclohexyl-carbodiimide / toluene / 2 h / Ambient temperature 5: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme |
-
-
99470-01-6
ethyl 4-cyanomethyl-2-ethoxy-benzoate

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: HCl / Heating 2: 59 percent / NaOH / ethanol / 1.5 h / 23 - 25 °C 3: 29 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 4: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme | |
| Multi-step reaction with 5 steps 1: HCl / Heating 2: 59 percent / NaOH / ethanol / 1.5 h / 23 - 25 °C 3: 85 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 5: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme | |
| Multi-step reaction with 4 steps 1: HCl / Heating 2: 59 percent / NaOH / ethanol / 1.5 h / 23 - 25 °C 3: 59 percent / N,N'-dicyclohexyl-carbodiimide / toluene / 2 h / Ambient temperature 4: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme |
-
-
147770-03-4
3-methyl-1-(2-piperidin-1-yl-phenyl)-butan-1-one

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 63 percent / Et3N, TiCl4 / toluene / Ambient temperature 2: 76 percent / H2, Ti(O-iPr)4 / Raney-Ni / ethanol / 72 h / 50 °C / 150012 Torr 3: H2, aq. HCl / 10 percent Pd/C / H2O / 5 h / 50 °C / 3750.3 Torr 4: 29 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 5: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme | |
| Multi-step reaction with 5 steps 1: Et3N, TiCl4 / toluene / Ambient temperature 2: 66 percent / H2, Ti(O-iPr)4 / Raney-Ni / ethanol / 160 h / 160 °C / 150012 Torr 3: H2, aq. HCl / 10 percent Pd/C / 10 h / 50 °C / 3750.3 Torr 4: 29 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 5: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme |

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 39 percent / aq. NaNO2, aq. HCl / 2 h / 35 °C 2: 29 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 3: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme | |
| Multi-step reaction with 4 steps 1: 39 percent / aq. NaNO2, aq. HCl / 2 h / 35 °C 2: 85 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 4: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 39 percent / aq. NaNO2, aq. HCl / 2 h / 35 °C 2: 59 percent / N,N'-dicyclohexyl-carbodiimide / toluene / 2 h / Ambient temperature 3: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme |
-
-
99469-99-5
2-(3-ethoxy-4-(ethoxycarbonyl)phenyl) acetic acid

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 29 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 2: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 85 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 3: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 59 percent / N,N'-dicyclohexyl-carbodiimide / toluene / 2 h / Ambient temperature 2: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme |
-
-
332347-69-0
ethyl 2-ethoxy-4-(2-ethoxy-2-oxoethyl)benzoate

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 59 percent / NaOH / ethanol / 1.5 h / 23 - 25 °C 2: 29 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 3: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme | |
| Multi-step reaction with 4 steps 1: 59 percent / NaOH / ethanol / 1.5 h / 23 - 25 °C 2: 85 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 4: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 59 percent / NaOH / ethanol / 1.5 h / 23 - 25 °C 2: 59 percent / N,N'-dicyclohexyl-carbodiimide / toluene / 2 h / Ambient temperature 3: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme |
-
-
147769-98-0
N-acetyl-N-<(S)-3-methyl-1-(2-(1-piperidinyl)phenyl)-1-butyl>-amine

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: aq. HCl / 5.5 h / Heating 2: 29 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 3: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme |
-
-
147769-97-9
N-acetyl-N-<3-methyl-1-(2-(1-piperidinyl)phenyl)-1-(Z)-buten-1-yl>-amine

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: H2, NaOH, Et3N, Ti(OiPr)4 / Ru(OAc)2<(S)-BINAP> / methanol; CH2Cl2 / 170 h / 30 °C / 75006 Torr 2: aq. HCl / 5.5 h / Heating 3: 29 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 4: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme |

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: Ti(OiPr)4 / tetrahydrofuran; toluene / 60 h / 100 °C 2: H2, aq. HCl / 10 percent Pd/C / 5 h / 50 °C / 3750.3 Torr 3: 29 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 4: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme |
-
-
219922-07-3
N-<(S)-3-methyl-1-<2-(1-piperidinyl)phenyl>butyl>-N-<(R')-1-phenethyl>-amine

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: H2, aq. HCl / 10 percent Pd/C / 5 h / 50 °C / 3750.3 Torr 2: 29 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 3: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme |
-
-
147770-01-2
N-<(S)-3-methyl-1-<2-(1-pieridinyl)phenyl>butyl>-N-<(S')-1-phenethyl>-amine

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: H2, aq. HCl / 10 percent Pd/C / H2O / 5 h / 50 °C / 3750.3 Torr 2: 29 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 3: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme |
-
-
219921-97-8
N-<(R)-3-methyl-1-<2-(1-piperidinyl)phenyl>butyl>-N-<(R')-1-phenethyl>-amine

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: H2, aq. HCl / 10 percent Pd/C / 10 h / 50 °C / 3750.3 Torr 2: 29 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 3: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme |

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 66 percent / H2, Ti(O-iPr)4 / Raney-Ni / ethanol / 160 h / 160 °C / 150012 Torr 2: H2, aq. HCl / 10 percent Pd/C / 10 h / 50 °C / 3750.3 Torr 3: 29 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 4: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme |

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 76 percent / H2, Ti(O-iPr)4 / Raney-Ni / ethanol / 72 h / 50 °C / 150012 Torr 2: H2, aq. HCl / 10 percent Pd/C / H2O / 5 h / 50 °C / 3750.3 Torr 3: 29 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 4: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme |

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 1.) LDA / 1.) THF, hexane, -20 deg C, 30 min, 2.) THF, hexane, from -70 deg C to RT, overnight 2: HCl / 0.5 h / 0 °C 3: Ambient temperature 4: Ti(OiPr)4 / tetrahydrofuran; toluene / 60 h / 100 °C 5: H2, aq. HCl / 10 percent Pd/C / 5 h / 50 °C / 3750.3 Torr 6: 29 percent / PPh3, Et3N, CCl4 / acetonitrile / 15 h / Ambient temperature 7: 92 percent / 1N NaOH / ethanol / 4 h / 60 °C View Scheme |

| Conditions | Yield |
|---|---|
| With 3-methyl-1-butyl acetate In chloroform at 65℃; Temperature; | 95.2% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol at 60 - 70℃; pH=7 - 8; | 91% |
-
-
80463-22-5
4-(1-hydroxyethyl)benzyl alcohol

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; | 90% |
-
-
1013027-15-0
1-(3-(hydroxymethyl)phenyl)ethan-1-ol

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; | 90% |
-
-
931-53-3
Cyclohexyl isocyanide

-
-
100-52-7
benzaldehyde

-
-
108-44-1
1-amino-3-methylbenzene

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 6h; Ugi Condensation; | 87% |

-
-
931-53-3
Cyclohexyl isocyanide

-
-
104-88-1
4-chlorobenzaldehyde

-
-
62-53-3
aniline

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 6h; Ugi Condensation; | 85% |

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| With dipotassium hydrogenphosphate; fac-tris(2-phenylpyridinato-N,C2')iridium(III); tris-(trimethylsilyl)silane; dimethyl dicarbonate In acetonitrile at 20℃; for 24h; Schlenk technique; Inert atmosphere; Sealed tube; Irradiation; | 84% |
-
-
931-53-3
Cyclohexyl isocyanide

-
-
104-87-0
4-methyl-benzaldehyde

-
-
108-44-1
1-amino-3-methylbenzene

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 6h; Ugi Condensation; | 83% |


| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol; water for 2h; pH=9; | 82% |
-
-
931-53-3
Cyclohexyl isocyanide

-
-
104-87-0
4-methyl-benzaldehyde

-
-
62-53-3
aniline

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 6h; Ugi Condensation; | 82% |

-
-
762-04-9
phosphonic acid diethyl ester

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| With palladium diacetate; 2,2-dimethylpropanoic anhydride; triethylamine; 1,4-di(diphenylphosphino)-butane In 1,4-dioxane at 160℃; for 15h; Inert atmosphere; Schlenk technique; | 80% |
-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| With 2C11H15O2(1-)*C10H14*Ru(2+); water-d2 In 1,4-dioxane at 100℃; for 16h; | 80% |
-
-
931-53-3
Cyclohexyl isocyanide

-
-
106-47-8
4-chloro-aniline

-
-
105-07-7
4-cyanobenzaldehyde

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 6h; Ugi Condensation; | 80% |


| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 6h; Ugi Condensation; | 78% |

-
-
135062-02-1
(S)-repaglinide

-
-
108-95-2
phenol

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In tetrahydrofuran at 50℃; Inert atmosphere; | 77% |
-
-
931-53-3
Cyclohexyl isocyanide

-
-
555-16-8
4-nitrobenzaldehdye

-
-
62-53-3
aniline

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 6h; Ugi Condensation; | 77% |


-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| With potassium phosphate; dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer; silver(I) acetate In acetonitrile at 80℃; for 24h; Sealed tube; Molecular sieve; | 77% |
-
-
931-53-3
Cyclohexyl isocyanide

-
-
104-87-0
4-methyl-benzaldehyde

-
-
106-47-8
4-chloro-aniline

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 6h; Ugi Condensation; | 76% |


| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 5h; Inert atmosphere; | 75% |
-
-
931-53-3
Cyclohexyl isocyanide

-
-
62-53-3
aniline

-
-
105-07-7
4-cyanobenzaldehyde

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 6h; Ugi Condensation; | 75% |


| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol for 3h; pH=8; | 74% |

-
-
931-53-3
Cyclohexyl isocyanide

-
-
123-11-5
4-methoxy-benzaldehyde

-
-
62-53-3
aniline

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 6h; Ugi Condensation; | 74% |

-
-
931-53-3
Cyclohexyl isocyanide

-
-
100-52-7
benzaldehyde

-
-
106-47-8
4-chloro-aniline

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 6h; Ugi Condensation; | 73% |

-
-
931-53-3
Cyclohexyl isocyanide

-
-
99-61-6
3-nitro-benzaldehyde

-
-
62-53-3
aniline

-
-
135062-02-1
(S)-repaglinide

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 6h; Ugi Condensation; | 72% |

Repaglinide Chemical Properties
CAS NO: 135062-02-1
Molecular Formula: C27H36N2O4
Molecular Weight: 452.585740 g/mol
Index of Refraction: 1.567
Surface Tension: 47 dyne/cm
Density: 1.137 g/cm3
Flash Point: 360.8 °C
Enthalpy of Vaporization: 103.84 kJ/mol
Boiling Point: 672.9 °C at 760 mmHg
Vapour Pressure: 5E-19 mmHg at 25°C
Melting Point: 129-130.2 °C
Storage temp: 2-8°C
Solubility DMSO: 34 mg/mL
Appearance: White to off-white solid
Molecular Structure: 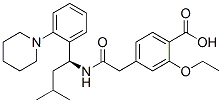
IUPAC Name: 2-Ethoxy-4-[2-[[(1S)-3-methyl-1-(2-piperidin-1-ylphenyl)butyl]amino]-2-oxoethyl]benzoic acid
Synonyms of Repaglinide (CAS NO.135062-02-1) : (+)-2-Ethoxy-alpha-(((S)-alpha-isobutyl-o-piperidinobenzyl)carbamoyl)-p-toluic acid ; (S)-2-Ethoxy-4-(2-((methyl-1-(2-(1-piperidinyl)phenyl)butyl)amino)-2-oxoethyl)-benzoic acid ; Prandin ; Repaglinida ; Repaglinidum ; Benzoic acid, 2-ethoxy-4-(2-((3-methyl-1-(2-(1-piperidinyl)phenyl)butyl)amino)-2-oxoethyl)-, (S)-
Product Categories: Pharmaceutical material and intermeidates;Active Pharmaceutical Ingredients;Chiral Reagents;Amino Acids 13C, 2H, 15N;Chemical Impurities;Impurities;Intermediates & Fine Chemicals;Pharmaceuticals;Amino Acids & Derivatives
Repaglinide History
The drug was the first of the meglitinide class, which was later licensed by Boehringer to Novo Nordisk, which filed an Investigational New Drug application for the compound with the Food and Drug Administration (FDA) in April 1992. Novo Nordisk filed its New Drug Application (NDA) for Prandin in July 1997 and it was quickly approved, gaining FDA approval in December 1997. . Repaglinide (CAS NO.135062-02-1) was branded Prandin because its quick onset and short duration of action concentrates its effect around meal time (the prandium was the Roman meal which is comparable to the modern lunch).
Repaglinide Uses
Repaglinide (CAS NO.135062-02-1) is used for the treatment of type II diabetes. It belongs to the meglitinide class of blood glucose-lowering drugs.
Repaglinide Safety Profile
WGK Germany: 2
RTECS: 000000033825
Repaglinide Specification
Repaglinide (CAS NO.135062-02-1) should not be administered concomitantly with gemfibrozil, clarithromycin or azole antifungals such as itraconazole or ketoconazole.Administration of both repaglinide and one or more of these drugs results in an increase in plasma concentration of repaglinide and may lead to hypoglycemia.
Related Products
- Repaglinide
- 135064-15-2
- 135065-69-9
- 135065-71-3
- 135065-76-8
- 135066-21-6
- 13506-76-8
- 13506-78-0
- 135072-14-9
- 135072-15-0
- 135072-21-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View