-
Name
Retinol palmitate
- EINECS 201-228-5
- CAS No. 79-81-2
- Article Data19
- CAS DataBase
- Density 0.92 g/cm3
- Solubility Soluble in chloroform, ether, and vegetable oils. Insoluble in water.
- Melting Point 2-8 °C
- Formula C36H60O2
- Boiling Point 607.5 °C at 760 mmHg
- Molecular Weight 524.871
- Flash Point 79.7 °C
- Transport Information
- Appearance
- Safety 53-23-36/37/39-45-36/37
- Risk Codes 63
-
Molecular Structure
-
Hazard Symbols
 T,
T, Xn
Xn
- Synonyms Retinolpalmitate (6CI,7CI);Retinol, palmitate, all-trans- (8CI);250S/N;250S/N-B;Aquapalm;Aquasol A;Arovit;Arovit (Roche);Axerophthol palmitate;DispatabsTabs;Lutavit A 500 Plus;Optovit A;Palmitic acid, ester withretinol;Retinyl palmitate;Testavol S;VAP 250CK-CWD;VAP 250MS-CWD;Vitamin Apalmitate;Vitazyme A;all-trans-Retinol palmitate;all-trans-Vitamin A palmitate;trans-Retinyl palmitate;Vitamin A palmitate;
- PSA 26.30000
- LogP 11.54250
Synthetic route

| Conditions | Yield |
|---|---|
| In toluene at 35℃; for 1h; | 99% |
| With pyridine In 1,2-dichloro-ethane |

| Conditions | Yield |
|---|---|
| With aluminum oxide In Petroleum ether at 40℃; for 2h; Solvent; | 97.62% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol at 55℃; under 11.2511 - 16.5017 Torr; for 3h; Concentration; Pressure; Large scale; | 96% |

| Conditions | Yield |
|---|---|
| at 60℃; under 0.750075 Torr; for 3h; Temperature; Large scale; | 94% |

-
-
62285-98-7
5-(2,6,6-trimethylcyclohexenyl)-3-methyl-2,4-pentadienyltriphenylphosphonium bromide

-
-
79-81-2
retinyl palmitate

| Conditions | Yield |
|---|---|
| With sodium ethanolate In N,N-dimethyl-formamide at -5 - 5℃; for 3h; Wittig Olefination; Inert atmosphere; | 91.3% |

-
-
53282-28-3
[(2E,4E)-3-methyl-5-(2,6,6-trimethylcyclohex-1-en-1-yl)penta-2,4-dienyl]triphenylphosphonium chloride

-
-
79-81-2
retinyl palmitate

| Conditions | Yield |
|---|---|
| With potassium hydroxide In isopropyl alcohol at 5 - 20℃; for 2h; Wittig Olefination; Inert atmosphere; | 90.8% |
-
-
186194-14-9
diethyl 3-methyl-5-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4-pentadienylphosphonate

-
-
79-81-2
retinyl palmitate

| Conditions | Yield |
|---|---|
| Stage #1: diethyl 3-methyl-5-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4-pentadienylphosphonate With sodium t-butanolate In toluene at 0℃; for 0.5h; Inert atmosphere; Stage #2: 2-methyl-4-palmitoyloxy-2-butenal In toluene for 3h; Reagent/catalyst; Solvent; Inert atmosphere; | 89% |
-
-
127-47-9
Retinol acetate

-
-
57-10-3
1-hexadecylcarboxylic acid

-
A
-
79-81-2
retinyl palmitate

-
B
-
68-26-8
RETINOL

| Conditions | Yield |
|---|---|
| With Novozyme 435 (from Candida antarctica immobilized on acrylic resin); Amberlyst A-21 In toluene at 20℃; for 15h; Product distribution / selectivity; Enzymatic reaction; | A 78% B n/a |


| Conditions | Yield |
|---|---|
| With 1,2-dichloro-ethane |

-
-
110-86-1
pyridine

-
-
108-88-3
toluene

-
-
10025-87-3, 12599-09-6, 63736-95-8
trichlorophosphate

-
-
79-81-2
retinyl palmitate

| Conditions | Yield |
|---|---|
| at 90 - 95℃; |


-
-
128759-92-2
3-methyl-5-(2,6,6-trimethyl-1-cyclohexen-1-yl)-1,4-pentadienylphosphonic acid,diethyl ester

-
-
79-81-2
retinyl palmitate

| Conditions | Yield |
|---|---|
| With sodium t-butanolate In water; N,N-dimethyl-formamide; toluene |

| Conditions | Yield |
|---|---|
| With Candida sp.99-125 lipase immobilized on SBA-15 In hexane at 30℃; for 6h; Enzymatic reaction; | 75.2 %Chromat. |
| With novozyme 435 In toluene at 20℃; for 20h; Schlenk technique; Inert atmosphere; Enzymatic reaction; |
-
-
82010-11-5
farnesyl alcohol

-
-
79-81-2
retinyl palmitate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: TEMPOL; copper(l) chloride / N,N-dimethyl-formamide / 2 h / 30 °C 2.1: 2,3-dicyano-5,6-dichloro-p-benzoquinone / toluene / 0.5 h / 50 °C 3.1: sulfuric acid / 1,2-dichloro-ethane / 0 - 5 °C 4.1: triphenylphosphine / methanol / 1 h / 45 °C 4.2: 1 h / 45 °C 5.1: toluene / 1 h / 35 °C View Scheme |
-
-
80442-43-9
(2E)-farnesal

-
-
79-81-2
retinyl palmitate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: 2,3-dicyano-5,6-dichloro-p-benzoquinone / toluene / 0.5 h / 50 °C 2.1: sulfuric acid / 1,2-dichloro-ethane / 0 - 5 °C 3.1: triphenylphosphine / methanol / 1 h / 45 °C 3.2: 1 h / 45 °C 4.1: toluene / 1 h / 35 °C View Scheme |
-
-
13832-89-8, 64197-46-2, 74284-77-8, 83378-81-8, 83378-82-9, 83378-83-0, 83378-84-1
(2E,4E,6Z)-3,7,11-Trimethyl-dodeca-2,4,6,10-tetraenal

-
-
79-81-2
retinyl palmitate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: sulfuric acid / 1,2-dichloro-ethane / 0 - 5 °C 2.1: triphenylphosphine / methanol / 1 h / 45 °C 2.2: 1 h / 45 °C 3.1: toluene / 1 h / 35 °C View Scheme |
-
-
3917-41-7
(2E,4E)-3-methyl-5-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4-pentadienal

-
-
79-81-2
retinyl palmitate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: triphenylphosphine / methanol / 1 h / 45 °C 1.2: 1 h / 45 °C 2.1: toluene / 1 h / 35 °C View Scheme |
-
-
59633-88-4
2-(bromomethyl)-1,3,3-trimethylcyclohex-1-ene

-
-
79-81-2
retinyl palmitate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: hexane / 4 h / 50 - 55 °C / Inert atmosphere 2.1: potassium hydroxide / isopropyl alcohol / 1 h / -5 - 5 °C / Inert atmosphere 3.1: potassium hydroxide / water; dichloromethane / 3 h / 10 - 15 °C 3.2: 3 h / 20 - 25 °C 4.1: isopropyl alcohol / 5 h / 55 - 60 °C / Inert atmosphere 5.1: potassium hydroxide / isopropyl alcohol / 2 h / 5 - 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 5 steps 1.1: hexane / 4 h / 50 - 55 °C / Inert atmosphere 2.1: potassium hydroxide / isopropyl alcohol / 1 h / -5 - 5 °C / Inert atmosphere 3.1: sodium hydroxide / water; dichloromethane / 3 h / 15 - 20 °C 3.2: 3 h / 20 - 25 °C 4.1: acetonitrile / 5 h / 50 - 55 °C / Inert atmosphere 5.1: sodium ethanolate / N,N-dimethyl-formamide / 3 h / -5 - 5 °C / Inert atmosphere View Scheme |

-
-
79-81-2
retinyl palmitate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: sodium ethanolate / N,N-dimethyl-formamide / 5 h / -5 - 10 °C / Inert atmosphere 2.1: potassium hydroxide / water; dichloromethane / 3 h / 10 - 15 °C 2.2: 3 h / 20 - 25 °C 3.1: isopropyl alcohol / 5 h / 55 - 60 °C / Inert atmosphere 4.1: potassium hydroxide / isopropyl alcohol / 2 h / 5 - 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 4 steps 1.1: sodium ethanolate / N,N-dimethyl-formamide / 5 h / -5 - 10 °C / Inert atmosphere 2.1: sodium hydroxide / water; dichloromethane / 3 h / 15 - 20 °C 2.2: 3 h / 20 - 25 °C 3.1: acetonitrile / 5 h / 50 - 55 °C / Inert atmosphere 4.1: sodium ethanolate / N,N-dimethyl-formamide / 3 h / -5 - 5 °C / Inert atmosphere View Scheme |

-
-
79-81-2
retinyl palmitate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: sodium methylate / N,N-dimethyl-formamide / 1 h / 0 - 15 °C / Inert atmosphere 2.1: potassium hydroxide / water; dichloromethane / 3 h / 10 - 15 °C 2.2: 3 h / 20 - 25 °C 3.1: isopropyl alcohol / 5 h / 55 - 60 °C / Inert atmosphere 4.1: potassium hydroxide / isopropyl alcohol / 2 h / 5 - 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 4 steps 1.1: sodium methylate / N,N-dimethyl-formamide / 1 h / 0 - 15 °C / Inert atmosphere 2.1: sodium hydroxide / water; dichloromethane / 3 h / 15 - 20 °C 2.2: 3 h / 20 - 25 °C 3.1: acetonitrile / 5 h / 50 - 55 °C / Inert atmosphere 4.1: sodium ethanolate / N,N-dimethyl-formamide / 3 h / -5 - 5 °C / Inert atmosphere View Scheme |
-
-
56013-01-5
triphenyl((2,6,6-trimethylcyclohex-1-enyl)methyl)phosphonium bromide

-
-
79-81-2
retinyl palmitate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: potassium hydroxide / isopropyl alcohol / 1 h / -5 - 5 °C / Inert atmosphere 2.1: potassium hydroxide / water; dichloromethane / 3 h / 10 - 15 °C 2.2: 3 h / 20 - 25 °C 3.1: isopropyl alcohol / 5 h / 55 - 60 °C / Inert atmosphere 4.1: potassium hydroxide / isopropyl alcohol / 2 h / 5 - 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 4 steps 1.1: potassium hydroxide / isopropyl alcohol / 1 h / -5 - 5 °C / Inert atmosphere 2.1: sodium hydroxide / water; dichloromethane / 3 h / 15 - 20 °C 2.2: 3 h / 20 - 25 °C 3.1: acetonitrile / 5 h / 50 - 55 °C / Inert atmosphere 4.1: sodium ethanolate / N,N-dimethyl-formamide / 3 h / -5 - 5 °C / Inert atmosphere View Scheme |
-
-
3917-38-2
3-methyl-5-acetoxy-1-(2,6,6-trimethyl-1-cyclohexen-1-yl)-1,3-pentadiene

-
-
79-81-2
retinyl palmitate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: potassium hydroxide / water; dichloromethane / 3 h / 10 - 15 °C 1.2: 3 h / 20 - 25 °C 2.1: isopropyl alcohol / 5 h / 55 - 60 °C / Inert atmosphere 3.1: potassium hydroxide / isopropyl alcohol / 2 h / 5 - 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1.1: sodium hydroxide / water; dichloromethane / 3 h / 15 - 20 °C 1.2: 3 h / 20 - 25 °C 2.1: acetonitrile / 5 h / 50 - 55 °C / Inert atmosphere 3.1: sodium ethanolate / N,N-dimethyl-formamide / 3 h / -5 - 5 °C / Inert atmosphere View Scheme |
-
-
55732-70-2
5-(2,6,6-trimethyl-cyclohex-1-enyl)-3-methyl-1-chloro-penta-2,4-diene

-
-
79-81-2
retinyl palmitate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: isopropyl alcohol / 5 h / 55 - 60 °C / Inert atmosphere 2: potassium hydroxide / isopropyl alcohol / 2 h / 5 - 20 °C / Inert atmosphere View Scheme |
-
-
38987-92-7
3-methyl-5-bromo-1-(2,6,6-trimethyl-1-cyclohexen-1-yl)-1,3-pentadiene

-
-
79-81-2
retinyl palmitate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: acetonitrile / 5 h / 50 - 55 °C / Inert atmosphere 2: sodium ethanolate / N,N-dimethyl-formamide / 3 h / -5 - 5 °C / Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: hydrogen bromide; sodium hydroxide / water; dichloromethane / 2 h / 30 - 35 °C / Inert atmosphere 1.2: 2 h / 35 - 40 °C 2.1: hexane / 4 h / 50 - 55 °C / Inert atmosphere 3.1: potassium hydroxide / isopropyl alcohol / 1 h / -5 - 5 °C / Inert atmosphere 4.1: sodium hydroxide / water; dichloromethane / 3 h / 15 - 20 °C 4.2: 3 h / 20 - 25 °C 5.1: acetonitrile / 5 h / 50 - 55 °C / Inert atmosphere 6.1: sodium ethanolate / N,N-dimethyl-formamide / 3 h / -5 - 5 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 6 steps 1.1: hydrogen bromide; sodium hydroxide / water; dichloromethane / 2 h / 30 - 35 °C / Inert atmosphere 1.2: 2 h / 35 - 40 °C 2.1: hexane / 4 h / 50 - 55 °C / Inert atmosphere 3.1: potassium hydroxide / isopropyl alcohol / 1 h / -5 - 5 °C / Inert atmosphere 4.1: potassium hydroxide / water; dichloromethane / 3 h / 10 - 15 °C 4.2: 3 h / 20 - 25 °C 5.1: isopropyl alcohol / 5 h / 55 - 60 °C / Inert atmosphere 6.1: potassium hydroxide / isopropyl alcohol / 2 h / 5 - 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 6 steps 1.1: sodium hydroxide; thionyl chloride / 1,2-dichloro-ethane; water / 3 h / 40 - 45 °C 1.2: 5 h / 70 - 75 °C 2.1: acetonitrile / 4 h / 60 - 65 °C / Inert atmosphere 3.1: sodium ethanolate / N,N-dimethyl-formamide / 5 h / -5 - 10 °C / Inert atmosphere 4.1: sodium hydroxide / water; dichloromethane / 3 h / 15 - 20 °C 4.2: 3 h / 20 - 25 °C 5.1: acetonitrile / 5 h / 50 - 55 °C / Inert atmosphere 6.1: sodium ethanolate / N,N-dimethyl-formamide / 3 h / -5 - 5 °C / Inert atmosphere View Scheme |

-
-
79-81-2
retinyl palmitate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: acetonitrile / 4 h / 60 - 65 °C / Inert atmosphere 2.1: sodium ethanolate / N,N-dimethyl-formamide / 5 h / -5 - 10 °C / Inert atmosphere 3.1: sodium hydroxide / water; dichloromethane / 3 h / 15 - 20 °C 3.2: 3 h / 20 - 25 °C 4.1: acetonitrile / 5 h / 50 - 55 °C / Inert atmosphere 5.1: sodium ethanolate / N,N-dimethyl-formamide / 3 h / -5 - 5 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 5 steps 1.1: acetonitrile / 4 h / 60 - 65 °C / Inert atmosphere 2.1: sodium ethanolate / N,N-dimethyl-formamide / 5 h / -5 - 10 °C / Inert atmosphere 3.1: potassium hydroxide / water; dichloromethane / 3 h / 10 - 15 °C 3.2: 3 h / 20 - 25 °C 4.1: isopropyl alcohol / 5 h / 55 - 60 °C / Inert atmosphere 5.1: potassium hydroxide / isopropyl alcohol / 2 h / 5 - 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 5 steps 1.1: 4 h / 105 - 110 °C / Inert atmosphere 2.1: sodium methylate / N,N-dimethyl-formamide / 1 h / 0 - 15 °C / Inert atmosphere 3.1: potassium hydroxide / water; dichloromethane / 3 h / 10 - 15 °C 3.2: 3 h / 20 - 25 °C 4.1: isopropyl alcohol / 5 h / 55 - 60 °C / Inert atmosphere 5.1: potassium hydroxide / isopropyl alcohol / 2 h / 5 - 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 5 steps 1.1: 4 h / 105 - 110 °C / Inert atmosphere 2.1: sodium methylate / N,N-dimethyl-formamide / 1 h / 0 - 15 °C / Inert atmosphere 3.1: sodium hydroxide / water; dichloromethane / 3 h / 15 - 20 °C 3.2: 3 h / 20 - 25 °C 4.1: acetonitrile / 5 h / 50 - 55 °C / Inert atmosphere 5.1: sodium ethanolate / N,N-dimethyl-formamide / 3 h / -5 - 5 °C / Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: magnesium chloride; di-tert-butyl dicarbonate / ethyl acetate / 5 h / 25 - 35 °C 2.1: selenium(IV) oxide; tert.-butylhydroperoxide / 18 h / 25 - 30 °C / Inert atmosphere 3.1: sodium t-butanolate / toluene / 0.5 h / 0 °C / Inert atmosphere 3.2: 3 h / Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: magnesium chloride; di-tert-butyl dicarbonate / ethyl acetate / 5 h / 25 - 35 °C 2.1: selenium(IV) oxide; tert.-butylhydroperoxide / 18 h / 25 - 30 °C / Inert atmosphere 3.1: sodium t-butanolate / toluene / 0.5 h / 0 °C / Inert atmosphere 3.2: 3 h / Inert atmosphere View Scheme |
-
-
79-81-2
retinyl palmitate

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol; isopropyl alcohol at 90℃; for 1.25h; | 90% |
-
-
79-81-2
retinyl palmitate

| Conditions | Yield |
|---|---|
| at 80℃; for 120h; Kinetics; Further Variations:; Solvents; Temperatures; Dimerization; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 90 percent / aq. 10percent KOH / propan-2-ol; ethanol / 1.25 h / 90 °C 2: 58 percent / MnO2, NaCN / acetonitrile; acetic acid / 62 h / 20 °C 3: aq. 5percent KOH / ethanol / 0.5 h / Heating View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 90 percent / aq. 10percent KOH / propan-2-ol; ethanol / 1.25 h / 90 °C 2: 58 percent / MnO2, NaCN / acetonitrile; acetic acid / 62 h / 20 °C 3: 77 percent / aq. 5percent KOH / ethanol / 0.5 h / Heating View Scheme |
-
-
79-81-2
retinyl palmitate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 90 percent / aq. 10percent KOH / propan-2-ol; ethanol / 1.25 h / 90 °C 2: 58 percent / MnO2, NaCN / acetonitrile; acetic acid / 62 h / 20 °C View Scheme |
-
-
79-81-2
retinyl palmitate

-
-
138093-35-3
20,14-retro-Retinoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 90 percent / aq. 10percent KOH / propan-2-ol; ethanol / 1.25 h / 90 °C 2: 58 percent / MnO2, NaCN / acetonitrile; acetic acid / 62 h / 20 °C 3: 77 percent / aq. 5percent KOH / ethanol / 0.5 h / Heating 4: 1.) lithium diisopropylamide, HMPA / 1.) THF, hexane, -60 deg C, 5 min, 2.) THF, hexane, -70 deg C, 45 min View Scheme | |
| Multi-step reaction with 4 steps 1: 90 percent / aq. 10percent KOH / propan-2-ol; ethanol / 1.25 h / 90 °C 2: 58 percent / MnO2, NaCN / acetonitrile; acetic acid / 62 h / 20 °C 3: 77 percent / aq. 5percent KOH / ethanol / 0.5 h / Heating 4: 1.) n-BuLi, diisopropylamine / 1.) THF, hexane, 0 deg C, 10 min, 2.) THF, hexane, -60 deg C, 5 min View Scheme |
-
-
79-81-2
retinyl palmitate

-
-
138093-38-6
14-ethyl-20,14-retro-retinoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 90 percent / aq. 10percent KOH / propan-2-ol; ethanol / 1.25 h / 90 °C 2: 58 percent / MnO2, NaCN / acetonitrile; acetic acid / 62 h / 20 °C 3: 77 percent / aq. 5percent KOH / ethanol / 0.5 h / Heating 4: 1.) lithium diisopropylamide, HMPA / 1.) THF, hexane, -60 deg C, 5 min, 2.) THF, hexane, -70 deg C, 45 min View Scheme |
-
-
79-81-2
retinyl palmitate

-
-
138093-39-7
14-isopropyl-20,14-retro-retinoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 90 percent / aq. 10percent KOH / propan-2-ol; ethanol / 1.25 h / 90 °C 2: 58 percent / MnO2, NaCN / acetonitrile; acetic acid / 62 h / 20 °C 3: 77 percent / aq. 5percent KOH / ethanol / 0.5 h / Heating 4: 1.) lithium diisopropylamide, HMPA / 1.) THF, hexane, -60 deg C, 5 min, 2.) THF, hexane, -70 deg C, 45 min View Scheme |
-
-
79-81-2
retinyl palmitate

-
-
138093-44-4
methyl 14-ethyl-20,14-retro-retinoate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 90 percent / aq. 10percent KOH / propan-2-ol; ethanol / 1.25 h / 90 °C 2: 58 percent / MnO2, NaCN / acetonitrile; acetic acid / 62 h / 20 °C 3: 77 percent / aq. 5percent KOH / ethanol / 0.5 h / Heating 4: 1.) lithium diisopropylamide, HMPA / 1.) THF, hexane, -60 deg C, 5 min, 2.) THF, hexane, -70 deg C, 45 min 5: 77 percent / diethyl ether / 20 h / 0 °C View Scheme |
-
-
79-81-2
retinyl palmitate

-
-
138093-45-5
methyl 14-isopropyl-20,14-retro-retinoate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 90 percent / aq. 10percent KOH / propan-2-ol; ethanol / 1.25 h / 90 °C 2: 58 percent / MnO2, NaCN / acetonitrile; acetic acid / 62 h / 20 °C 3: 77 percent / aq. 5percent KOH / ethanol / 0.5 h / Heating 4: 1.) lithium diisopropylamide, HMPA / 1.) THF, hexane, -60 deg C, 5 min, 2.) THF, hexane, -70 deg C, 45 min 5: 89 percent / diethyl ether / 0.5 h / 0 °C View Scheme |
-
-
79-81-2
retinyl palmitate

-
-
138231-98-8
13-cis-14-Ethylretinoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 90 percent / aq. 10percent KOH / propan-2-ol; ethanol / 1.25 h / 90 °C 2: 58 percent / MnO2, NaCN / acetonitrile; acetic acid / 62 h / 20 °C 3: 77 percent / aq. 5percent KOH / ethanol / 0.5 h / Heating 4: 1.) lithium diisopropylamide, HMPA / 1.) THF, hexane, -60 deg C, 5 min, 2.) THF, hexane, -70 deg C, 45 min 5: 77 percent / diethyl ether / 20 h / 0 °C 6: 1.) MeONa, 2.) 10percent aq. KOH / 1.) MeOH, reflux, 7 h, 2.) EtOH, reflux View Scheme |
-
-
79-81-2
retinyl palmitate

-
-
138093-41-1
14-Ethylretinoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 90 percent / aq. 10percent KOH / propan-2-ol; ethanol / 1.25 h / 90 °C 2: 58 percent / MnO2, NaCN / acetonitrile; acetic acid / 62 h / 20 °C 3: 77 percent / aq. 5percent KOH / ethanol / 0.5 h / Heating 4: 1.) lithium diisopropylamide, HMPA / 1.) THF, hexane, -60 deg C, 5 min, 2.) THF, hexane, -70 deg C, 45 min 5: 77 percent / diethyl ether / 20 h / 0 °C 6: 1.) MeONa, 2.) 10percent aq. KOH / 1.) MeOH, reflux, 7 h, 2.) EtOH, reflux View Scheme |
-
-
79-81-2
retinyl palmitate

-
-
138231-99-9
13-cis-14-Isopropylretinoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 90 percent / aq. 10percent KOH / propan-2-ol; ethanol / 1.25 h / 90 °C 2: 58 percent / MnO2, NaCN / acetonitrile; acetic acid / 62 h / 20 °C 3: 77 percent / aq. 5percent KOH / ethanol / 0.5 h / Heating 4: 1.) lithium diisopropylamide, HMPA / 1.) THF, hexane, -60 deg C, 5 min, 2.) THF, hexane, -70 deg C, 45 min 5: 89 percent / diethyl ether / 0.5 h / 0 °C 6: 1.) NaH, 2.) 20percent aq. KOH, 18-crown-6 / 1.) DMF, 50 deg C, 90 min, 2.) EtOH, reflux View Scheme |
-
-
79-81-2
retinyl palmitate

-
-
138093-42-2
14-Isopropylretinoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 90 percent / aq. 10percent KOH / propan-2-ol; ethanol / 1.25 h / 90 °C 2: 58 percent / MnO2, NaCN / acetonitrile; acetic acid / 62 h / 20 °C 3: 77 percent / aq. 5percent KOH / ethanol / 0.5 h / Heating 4: 1.) lithium diisopropylamide, HMPA / 1.) THF, hexane, -60 deg C, 5 min, 2.) THF, hexane, -70 deg C, 45 min 5: 89 percent / diethyl ether / 0.5 h / 0 °C 6: 1.) NaH, 2.) 20percent aq. KOH, 18-crown-6 / 1.) DMF, 50 deg C, 90 min, 2.) EtOH, reflux View Scheme |
-
-
79-81-2
retinyl palmitate

-
-
138232-01-6
(2Z,4E,6E,8E)-2-Ethyl-3,7-dimethyl-9-(2,6,6-trimethyl-cyclohex-1-enyl)-nona-2,4,6,8-tetraenoic acid methyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 90 percent / aq. 10percent KOH / propan-2-ol; ethanol / 1.25 h / 90 °C 2: 58 percent / MnO2, NaCN / acetonitrile; acetic acid / 62 h / 20 °C 3: 77 percent / aq. 5percent KOH / ethanol / 0.5 h / Heating 4: 1.) lithium diisopropylamide, HMPA / 1.) THF, hexane, -60 deg C, 5 min, 2.) THF, hexane, -70 deg C, 45 min 5: 77 percent / diethyl ether / 20 h / 0 °C 6: MeONa / methanol / 7 h / Heating View Scheme |
Retinol palmitate Chemical Properties
Molecular structure of Vitamin A palmitate (CAS NO.79-81-2) is:
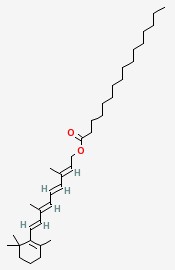
Product Name: Vitamin A palmitate
CAS Registry Number: 79-81-2
IUPAC Name: [(2E,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohexen-1-yl)nona-2,4,6,8-tetraenyl] hexadecanoate
Molecular Weight: 524.8604 [g/mol]
Molecular Formula: C36H60O2
XLogP3: 13.6
H-Bond Donor: 0
H-Bond Acceptor: 2
EINECS: 201-228-5
Melting Point: 2-8°C
Surface Tension: 35.1 dyne/cm
Density: 0.92 g/cm3
Flash Point: 79.7 °C
Enthalpy of Vaporization: 90.28 kJ/mol
Boiling Point: 607.5 °C at 760 mmHg
Vapour Pressure: 1.05E-14 mmHg at 25°C
Product Categories: Vitamins and derivatives
Retinol palmitate Uses
Vitamin A palmitate (CAS NO.79-81-2) is a synthetic alternate for retinyl acetate in vitamin A supplements, and is available in oily or dry forms. It is also a constituent of some topically-applied skin care products. Palmitate is an antioxidant and a vitamin A compound added to low fat milk and other dairy products to replace the vitamin content lost through the removal of milk fat. Palmitate is attached to the alcohol form of vitamin A, retinol, in order to make vitamin A stable in milk.
Retinol palmitate Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 4760mg/kg (4760mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" | Voprosy Onkologii. Problems of Oncology. For English translation, see PONCAU. Vol. 25(12), Pg. 84, 1979. |
| mouse | LD50 | oral | 6060mg/kg (6060mg/kg) | Journal of the American Academy of Dermatology. Vol. 6, Pg. 652, 1982. | |
| rat | LD50 | oral | 7910mg/kg (7910mg/kg) | Journal of the American Academy of Dermatology. Vol. 6, Pg. 652, 1982. |
Retinol palmitate Safety Profile
Hazard Codes:  T,
T,  Xn
Xn
Risk Statements: 63
R63:Possible risk of harm to the unborn child.
Safety Statements: 53-23-36/37/39-45-36/37
S53:Avoid exposure - obtain special instructions before use.
S23:Do not breathe vapour.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S36/37:Wear suitable protective clothing and gloves.
WGK Germany: 3
RTECS: VH6860000
F: 8-10-23
Retinol palmitate Specification
Vitamin A palmitate , its cas register number is 79-81-2. It also can be called Retinol palmitate ; Retinol, hexadecanoate ; Retinyl palmitate ; Retinol, all-trans-, palmitate ; Optovit-A ; Retinol, palmitate, all-trans- .
Related Products
- Retinol
- Retinol palmitate
- Retinol, 13-cis-
- 79814-40-7
- 79814-47-4
- 79815-20-6
- 79817-55-3
- 79817-57-5
- 79817-60-0
- 79817-61-1
- 79817-68-8
- 79817-75-7
- 79819-03-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View