-
Name
2-HYDROXYHIPPURIC ACID
- EINECS 207-661-6
- CAS No. 487-54-7
- Article Data10
- CAS DataBase
- Density 1.401 g/cm3
- Solubility soluble in water
- Melting Point 164-169 °C
- Formula C9H9NO4
- Boiling Point 489.79 °C at 760 mmHg
- Molecular Weight 195.175
- Flash Point 250.016 °C
- Transport Information
- Appearance light gray crystalline powder
- Safety 24/25-36/37/39-27-26
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Hippuricacid, o-hydroxy- (8CI);Salicyluric acid (6CI);(2-Hydroxybenzoyl)glycine;2-Hydroxyhippuric acid;N-(o-Hydroxybenzoyl)glycine;N-Salicyloylglycine;NSC524135;Salicyloylglycine;o-Hydroxyhippuric acid;
- PSA 86.63000
- LogP 0.59750
Synthetic route
-
-
178633-73-3
(2-Hydroxy-benzoylamino)-acetic acid benzyl ester

-
-
487-54-7
Salicyluric acid

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In methanol 0 deg C, then 0oom temp., overnight; | 92% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 20℃; | 90% |
| With sodium hydroxide; water Erwaermen des nach dem Ansaeuern erhaltenen Reaktionsprodukts mit wss. Natronlauge; |
-
-
27796-49-2
(2-methoxyphenyl)(methoxycarbonylmethylamino)-methanone

-
-
487-54-7
Salicyluric acid

| Conditions | Yield |
|---|---|
| 40% |

| Conditions | Yield |
|---|---|
| at 20℃; Kinetics; |
-
-
56145-98-3
N-salicyloyl-glycine amide

-
-
487-54-7
Salicyluric acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide |
-
-
856119-90-9
2-azidoacetoxy-benzoic acid

-
-
487-54-7
Salicyluric acid

| Conditions | Yield |
|---|---|
| With acetic acid methyl ester; platinum Hydrogenation; | |
| With palladium on activated charcoal; acetic acid Hydrogenation; |

-
-
487-54-7
Salicyluric acid

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; 2-methoxy-ethanol Hydrogenolyse; |
-
-
116601-30-0
2-(N-benzyloxycarbonyl-glycyloxy)-benzoic acid benzyl ester

-
-
487-54-7
Salicyluric acid

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; 2-methoxy-ethanol Hydrogenolyse; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide Behandeln des Reaktionsgemischs mit konz. Natronlauge; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide |

-
-
487-54-7
Salicyluric acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide for 2h; |
-
-
61141-14-8
anionic phenyl salicylate

-
-
56-40-6
glycine

-
A
-
487-54-7
Salicyluric acid

-
B
-
69-72-7
salicylic acid

-
C
-
108-95-2
phenol

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water; acetonitrile at 35℃; Rate constant; different contents of CH3CN; |

-
-
50331-25-4
(2,4-dioxo-4H-benzo[e][1,3]oxazin-3-yl)-acetic acid

-
-
487-54-7
Salicyluric acid

| Conditions | Yield |
|---|---|
| at 100℃; im Rohr; |

-
-
487-54-7
Salicyluric acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide |

-
-
487-54-7
Salicyluric acid

| Conditions | Yield |
|---|---|
| With water |

-
-
487-54-7
Salicyluric acid

| Conditions | Yield |
|---|---|
| With ammonium hydroxide at 100℃; im Bombenrohr; |
-
-
92505-60-7
2-methoxycarbonyloxy-benzoyl chloride

-
-
487-54-7
Salicyluric acid

| Conditions | Yield |
|---|---|
| With diethyl ether Verseifen des Reaktionsproduktes mit Natronlauge; |

| Conditions | Yield |
|---|---|
| With ethanol |


| Conditions | Yield |
|---|---|
| at 20℃; Kinetics; |

| Conditions | Yield |
|---|---|
| at 20℃; Kinetics; |
-
-
109-86-4
2-methoxy-ethanol

-
-
116601-30-0
2-(N-benzyloxycarbonyl-glycyloxy)-benzoic acid benzyl ester

-
-
487-54-7
Salicyluric acid

| Conditions | Yield |
|---|---|
| Hydrogenolyse; |


| Conditions | Yield |
|---|---|
| Hydrogenation; |


| Conditions | Yield |
|---|---|
| Hydrogenation; |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 72 percent / 1.) N-cyclohexyl-N'-<2-(4-methylmorpholin-4-ylium)ethyl>carboximide toluene-4-sulfonate; 2.) Et3N / acetonitrile / 1.) 30 min.; 2.) 0 deg C --> room temp., overnight 2: 92 percent / H2 / Pd/C / methanol / 0 deg C, then 0oom temp., overnight View Scheme |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 31 percent / chloroethylcarbonate / tetrahydrofuran 2: 40 percent View Scheme |
-
-
14216-34-3
2-methoxycarbonyloxy-benzoic acid

-
-
487-54-7
Salicyluric acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: PCl5 2: diethyl ether / Verseifen des Reaktionsproduktes mit Natronlauge View Scheme | |
| Multi-step reaction with 2 steps 1: PCl5 2: NaOH-solution / Behandeln des Reaktionsgemischs mit konz. Natronlauge View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: pyridine 2: palladium/charcoal; 2-methoxy-ethanol / Hydrogenolyse View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: dimethylaniline; benzene 2: PCl5 3: diethyl ether / Verseifen des Reaktionsproduktes mit Natronlauge View Scheme | |
| Multi-step reaction with 2 steps 1: PCl5; diethyl ether; pyridine 2: palladium/charcoal; 2-methoxy-ethanol / Hydrogenolyse View Scheme | |
| Multi-step reaction with 3 steps 1: dimethylaniline; benzene 2: PCl5 3: NaOH-solution / Behandeln des Reaktionsgemischs mit konz. Natronlauge View Scheme | |
| Multi-step reaction with 2 steps 1: chloroform; pyridine 2: platinum; methyl acetate / Hydrogenation View Scheme |
-
-
487-54-7
Salicyluric acid

-
-
108-24-7
acetic anhydride

-
-
99-61-6
3-nitro-benzaldehyde

-
-
16352-91-3
2-{4-[3-(nitrobenzylidene)-5-oxo-4,5-dihydrooxazol-2-yl]}phenyl acetate

| Conditions | Yield |
|---|---|
| With sodium acetate Erlenmeyer-Plochl-Bergmann; Reflux; | 88% |


| Conditions | Yield |
|---|---|
| With sodium acetate Erlenmeyer-Plochl-Bergmann; Reflux; | 88% |

-
-
487-54-7
Salicyluric acid

-
-
75755-40-7
2,2-dimethyl-3-(N-methyl-N-phenylamino)-2H-azirine

-
-
192046-51-8
2-Hydroxy-N-{[1-methyl-1-(methyl-phenyl-carbamoyl)-ethylcarbamoyl]-methyl}-benzamide

| Conditions | Yield |
|---|---|
| In acetonitrile 0 deg C --> room temp., overnight; | 86% |
-
-
487-54-7
Salicyluric acid

-
-
108-24-7
acetic anhydride

-
-
100-10-7
4-dimethylamino-benzaldehyde

-
-
16352-93-5
2-{4-[4-(dimethylamino)benzylidene]-5-oxo-4,5-dihydrooxazol-2-yl}phenyl acetate

| Conditions | Yield |
|---|---|
| With sodium acetate Erlenmeyer-Plochl-Bergmann; Reflux; | 85% |


| Conditions | Yield |
|---|---|
| With sodium acetate Erlenmeyer-Plochl-Bergmann; Reflux; | 85% |


| Conditions | Yield |
|---|---|
| With sodium acetate Erlenmeyer-Plochl-Bergmann; Reflux; | 85% |

-
-
487-54-7
Salicyluric acid

-
-
108-24-7
acetic anhydride

-
-
120-14-9
3,4-dimethoxy-benzaldehyde

-
-
16352-92-4
2-[4-(3,4-dimethoxybenzylidene)-5-oxo-4,5-dihydrooxazol-2-yl]phenyl acetate

| Conditions | Yield |
|---|---|
| With sodium acetate Erlenmeyer-Plochl-Bergmann; Reflux; | 80% |

-
-
487-54-7
Salicyluric acid

-
-
6038-19-3
3-aminodihydrothiophen-2(3H)-one hydrochloride

-
-
1258323-53-3
2-hydroxy-N-(2-oxo-2-(2-oxotetrahydrothiophen-3-ylamino)ethyl)benzamide

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; HATU In tetrahydrofuran at 60℃; for 1h; | 80% |

| Conditions | Yield |
|---|---|
| In acetone 0 deg C, 5 h then r.t., 48 h; | 70% |

-
-
487-54-7
Salicyluric acid

| Conditions | Yield |
|---|---|
| With triethylamine In methanol addn. of Et3N (1 equiv.) to ligand, addn. of Cu(ClO4)2 (0.5 equiv.), standing for 2-3 days (pptn.); collection (filtration), recrystn. (50% Me2CO); | 70% |

| Conditions | Yield |
|---|---|
| In acetone C3O2:acetone=1:500; 0 deg C, 4 h then r.t., 48 h; | 68% |
-
-
487-54-7
Salicyluric acid

-
-
56-17-7
cystamine dihydrochioride

-
-
1429128-83-5
N,N'-(2,2'-(2,2'-disulfanediylbis(ethane-2,1-diyl)bis(azanediyl))bis(2-oxoethane-2,1-diyl))bis(2-hydroxybenzamide)

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; HATU In tetrahydrofuran at 60℃; for 1h; | 65% |

| Conditions | Yield |
|---|---|
| With triethylamine In methanol addn. of Et3N (1 equiv.) to ligand, addn. to ZnCl2 (0.5 equiv.), stirring for 2 days (pptn); collection (filtration), drying (vac., over P2O5); elem. anal.; | 56% |

-
-
487-54-7
Salicyluric acid

| Conditions | Yield |
|---|---|
| With triethylamine In methanol addn. of Et3N (1 equiv.) to ligand, addn. to CoCl2 (0.5 equiv.), stirring for 24 h (pptn.); collection (filtration), drying (vac. over P2O5); elem. anal.; | 53% |

-
-
487-54-7
Salicyluric acid

| Conditions | Yield |
|---|---|
| With AcONa*3H2O; NaOH In ethanol; water N2-atmosphere; dropwise addn. of 1 equiv. VOSO4 to mixt. of ligand and AcONa (in 50% aq. EtOH), pH-adjustment to 4.5 (NaOH); standing for 1 week (pptn.), collection (filtration), washing (aq. EtOH), drying (vac.); elem. anal.; | 40% |

-
-
487-54-7
Salicyluric acid

| Conditions | Yield |
|---|---|
| With triethylamine In methanol addn. of Et3N (2 equiv.) into soln. of ligand, addn. to soln. of Cu(ClO4)2 (1 equiv.), stirring 6 h; collection (filtration), drying (60°C); elem. anal.; | 35% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride |
-
-
487-54-7
Salicyluric acid

-
-
108-24-7
acetic anhydride

-
-
100-52-7
benzaldehyde

-
-
16352-89-9
2-(2-acetoxy-phenyl)-4-((Z)-benzyliden)-4H-oxazol-5-one

| Conditions | Yield |
|---|---|
| With sodium acetate |

Salicyluric acid Specification
The Salicyluric acid with CAS registry number of 487-54-7 is also known as 2-Hydroxyhippuric acid. The IUPAC name is 2-[(2-Hydroxybenzoyl)amino]acetic acid. It belongs to product categories of Aromatics Compounds; Aromatics. Its EINECS registry number is 207-661-6. In addition, the formula is C9H9NO4 and the molecular weight is 195.17. This chemical is stable at normal temperature and pressure, and it should be sealed in a ventilated and dry place. What's more, it is a light gray crystalline powder.
Physical properties about Salicyluric acid are: (1)ACD/LogP: 0.81; (2)# of Rule of 5 Violations: 0 ; (3)ACD/BCF (pH 5.5): 1; (4)ACD/BCF (pH 7.4): 1; (5)ACD/KOC (pH 5.5): 1; (6)ACD/KOC (pH 7.4): 1; (7)#H bond acceptors: 5; (8)#H bond donors: 3; (9)#Freely Rotating Bonds: 4; (10)Index of Refraction: 1.604; (11)Molar Refractivity: 47.955 cm3; (12)Molar Volume: 139.342 cm3; (13)Surface Tension: 65.897 dyne/cm; (14)Density: 1.401 g/cm3; (15)Flash Point: 250.016 °C; (16)Enthalpy of Vaporization: 79.642 kJ/mol; (17)Boiling Point: 489.79 °C at 760 mmHg; (18)Vapour Pressure: 0 mmHg at 25 °C.
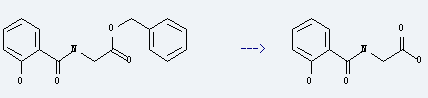
Preparation of Salicyluric acid: it is prepared by reaction of (2-hydroxy-benzoylamino)-acetic acid benzyl ester. The reaction needs reagent H2, catalyst Pd/C and solvent methanol at the temperature of 0 °C over night. The yield is about 92%.
Uses of Salicyluric acid: it is used to produce [(2-hydroxy-4,5-dioxo-4H,5H-pyrano[3,2-c]chromene-3-carbonyl)-amino]-acetic acid by reaction with propadienedione. The reaction occurs with solvent acetone at 0 °C for 5 hours and r.t. for 48 hours. The yield is about 70%.
![Salicyluric acid is used to produce [(2-hydroxy-4,5-dioxo-4H,5H-pyrano[3,2-c]chromene-3-carbonyl)-amino]-acetic acid by reaction with propadienedione.](/UserFilesUpload/Uses of Salicyluric acid.png)
When you are using this chemical, please be cautious about it. As a chemical, it is irritating to eyes, respiratory system and skin. During using it, wear suitable protective clothing, gloves and eye/face protection. Avoid contact with skin and eyes. After using it, take off immediately all contaminated clothing. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: C1=CC=C(C(=C1)C(=O)NCC(=O)O)O
2. InChI: InChI=1S/C9H9NO4/c11-7-4-2-1-3-6(7)9(14)10-5-8(12)13/h1-4,11H,5H2,(H,10,14)(H,12,13)
3. InChIKey: ONJSZLXSECQROL-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD | oral | > 1gm/kg (1000mg/kg) | Farmaco, Edizione Scientifica. Vol. 30, Pg. 399, 1975. | |
| mouse | LDLo | intravenous | 600mg/kg (600mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES CARDIAC: CHANGE IN RATE | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 38, Pg. 9, 1930. |
| pigeon | LDLo | intravenous | 2080mg/kg (2080mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD CARDIAC: CHANGE IN RATE LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 38, Pg. 9, 1930. |
| pigeon | LDLo | subcutaneous | 5730mg/kg (5730mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES CARDIAC: CHANGE IN RATE | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 38, Pg. 9, 1930. |
| rat | LD50 | subcutaneous | 3gm/kg (3000mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD CARDIAC: CHANGE IN RATE LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 38, Pg. 9, 1930. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View