-
Name
Terephthalic acid
- EINECS 262-050-1
- CAS No. 100-21-0
- Article Data717
- CAS DataBase
- Density 1.51 g/cm3
- Solubility slightly soluble in water (0,017 g/L at 25°C)
- Melting Point 300 °C
- Formula C8H6O4
- Boiling Point 392.4 °C at 760 mmHg
- Molecular Weight 166.133
- Flash Point 260°C
- Transport Information
- Appearance white powder
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Terephthalicacid (7CI,8CI);1,4-Dicarboxybenzene;4-Carboxybenzoic acid;Amoco TA 33;NSC36973;QTA;TA 33LP;WR 16262;p-Benzenedicarboxylic acid;p-Carboxybenzoic acid;p-Dicarboxybenzene;p-Phthalic acid;PTA;1,4-Benzenedicarboxylic acid;
- PSA 74.60000
- LogP 1.08300
Synthetic route

| Conditions | Yield |
|---|---|
| With ammonium acetate; water; hydrogen bromide; oxygen; manganese(II) acetate; cobalt(II) diacetate tetrahydrate; 1-n-butyl-3-methylimidazolim bromide; acetic acid at 200℃; under 30753.1 Torr; for 10h; Time; Temperature; Reagent/catalyst; Concentration; Inert atmosphere; | 99.9% |
| With oxovanadium(IV) sulfate; hydrogen bromide; oxygen; acetic acid In water at 100℃; under 750.075 Torr; for 20h; | 98% |
| With oxygen; acetic acid; hydrogen bromide; cobalt(II) acetate; manganese(II) acetate In water at 200℃; under 22502.3 Torr; for 0.05h; Product distribution / selectivity; Inert atmosphere; | 98.3% |

| Conditions | Yield |
|---|---|
| With ammonium acetate; water; hydrogen bromide; oxygen; manganese(II) acetate; cobalt(II) diacetate tetrahydrate; 1-n-butyl-3-methylimidazolim bromide; acetic acid; 3-butyl-1-methylimidazolium acetate at 215℃; under 30753.1 Torr; for 3h; Inert atmosphere; | 99.1% |
| With ammonium acetate; water; hydrogen bromide; oxygen; manganese(II) acetate; cobalt(II) diacetate tetrahydrate; 1-n-butyl-3-methylimidazolim bromide; acetic acid; 3-butyl-1-methylimidazolium acetate at 215℃; under 30753.1 Torr; for 3h; Inert atmosphere; | 99.1% |
| With oxovanadium(IV) sulfate; hydrogen bromide; oxygen; acetic acid In water at 100℃; under 750.075 Torr; for 20h; | 97% |

| Conditions | Yield |
|---|---|
| With air; hydrogen bromide; acetic acid; cobalt(II) acetate; manganese(II) acetate at 180℃; under 25745 Torr; for 0.166667h; | A 98.3% B 1.4% |
| With 9,10-Dibromoanthracene; acetic acid; cobalt(II) acetate; cerium(III) acetate at 165℃; under 25745 Torr; for 0.166667h; | A 98.2% B 1.1% |
| With 9,10-Dibromoanthracene; acetic acid; cobalt(II) acetate; manganese(II) acetate at 170℃; under 25745 Torr; for 0.166667h; | A 97.5% B 1.9% |
-
-
106-42-3
para-xylene

-
A
-
100-21-0
terephthalic acid

-
B
-
104-87-0
4-methyl-benzaldehyde

-
C
-
619-66-9
4-Carboxybenzaldehyde

-
D
-
99-94-5
p-Toluic acid

| Conditions | Yield |
|---|---|
| With hydrogen bromide; oxygen; acetic acid; cobalt(II) acetate; manganese(II) acetate In water at 190℃; under 16501.7 Torr; for 1h; Product distribution / selectivity; | A 98.1% B 0.2% C 0.4% D 0.4% |
| With oxygen; acetic acid; palladium diacetate; antimony(III) acetate In water at 182 - 195℃; under 16501.7 - 20929.4 Torr; for 1 - 1.5h; Product distribution / selectivity; | A 50.3% B 7.2% C 6.4% D 6.2% |
| With hydrogen bromide; oxygen; acetic acid; zirconium oxyacetate; cobalt(II) acetate In water at 190℃; under 16501.7 Torr; for 1h; Product distribution / selectivity; | A 4.9% B 3% C 1.9% D 36.9% |


| Conditions | Yield |
|---|---|
| With NADH oxidase and vanillin dehydrogenase 2 co-expressed in Escherichia coli cells In aq. phosphate buffer at 30℃; for 12h; pH=7; Microbiological reaction; | 98% |
| With tris[2-(4,6-difluorophenyl)pyridinato-C2,N]-iridium(III); oxygen In acetonitrile at 20℃; for 24h; Irradiation; Sealed tube; Green chemistry; chemoselective reaction; | 95% |
| With sodium perborate In acetic acid at 45 - 50℃; | 93% |

| Conditions | Yield |
|---|---|
| With water; ozone In acetonitrile for 5h; Irradiation; | 98% |
| With NADH oxidase and vanillin dehydrogenase 2 co-expressed in Escherichia coli cells In aq. phosphate buffer at 30℃; for 3h; pH=7; Microbiological reaction; | 98% |
| With C4H11FeMo6NO24(3-)*3C16H36N(1+); water; oxygen; sodium carbonate at 50℃; under 760.051 Torr; for 8h; Green chemistry; | 97% |

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; dicobalt octacarbonyl In sodium hydroxide at 65℃; for 8h; | 98% |
| With sodium hydroxide; dicobalt octacarbonyl In water at 65℃; under 1471.02 Torr; for 6h; Product distribution; Irradiation; | 93.4 % Chromat. |

| Conditions | Yield |
|---|---|
| With oxygen; 1-hydroxy-pyrrolidine-2,5-dione; cobalt(II) acetate In acetic acid at 60℃; under 12049.9 Torr; for 1h; Product distribution / selectivity; | 98% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; cobalt(II) acetate In ethanol at 30℃; under 1520 Torr; for 4h; Irradiation; | 97.1% |
| With sodium hydroxide; dicobalt octacarbonyl In ethanol; water at 65℃; under 1471.02 Torr; for 4h; Product distribution; Irradiation; | 82.5 % Chromat. |

| Conditions | Yield |
|---|---|
| With diethylene glycol dimethyl ether at 70℃; for 0.583333h; Sonication; | 97% |
| With C24H33IrN4O3; water; sodium hydroxide for 18h; Reflux; | 96% |
| With sodium hypochlorite; water at 25℃; for 0.5h; | 94% |

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; dicobalt octacarbonyl In sodium hydroxide; benzene at 65℃; for 1.5h; Irradiation; | 97% |
-
-
32555-96-7
4-styrylbenzaldehyde

-
-
100-21-0
terephthalic acid

| Conditions | Yield |
|---|---|
| Stage #1: 4-styrylbenzaldehyde With tert.-butylhydroperoxide; iron(III) chloride hexahydrate; sodium hydroxide In water at 80℃; for 10h; Stage #2: With hydrogenchloride In water at 20℃; | 97% |

| Conditions | Yield |
|---|---|
| With C24H33IrN4O3; water; sodium hydroxide for 18h; Reflux; | 97% |
| Multi-step reaction with 2 steps 1: recombinant 5-hydroxymethylfurfural oxidase / aq. phosphate buffer / 4 h / 25 °C / pH 8 / Enzymatic reaction 2: recombinant 5-hydroxymethylfurfural oxidase / aq. phosphate buffer / 1 h / 25 °C / pH 8 / Enzymatic reaction View Scheme | |
| Multi-step reaction with 2 steps 1: recombinant 5-hydroxymethylfurfural oxidase / aq. phosphate buffer / 4 h / 25 °C / pH 8 / Enzymatic reaction 2: recombinant 5-hydroxymethylfurfural oxidase / aq. phosphate buffer / 1 h / 25 °C / pH 8 / Enzymatic reaction View Scheme |

| Conditions | Yield |
|---|---|
| With PPA at 154℃; for 4.2h; | 96% |
| With potassium carbonate |

| Conditions | Yield |
|---|---|
| With N-hydroxyphthalimide; oxygen; nitric acid at 110℃; under 760.051 Torr; for 6h; Ionic liquid; | A 96% B 3% |
| Stage #1: para-xylene With N-hydroxyphthalimide; cobalt(II) phthalocyanine; μ-oxo[manganese(III) tetraphenylporphine]2; oxygen at 120℃; under 3750.38 Torr; Stage #2: With N-hydroxyphthalimide; cobalt(II) phthalocyanine; μ-oxo[manganese(III) tetraphenylporphine]2; oxygen; acetic acid at 232℃; under 16501.7 Torr; for 1.5h; Temperature; Pressure; Reagent/catalyst; | A 90% B 9.7% |
| With chromium(VI) oxide; periodic acid In acetonitrile at 20℃; for 1h; | A 6% B 86% |

-
-
100-21-0
terephthalic acid

| Conditions | Yield |
|---|---|
| Stage #1: polyethylene terephthalate With sodium hydroxide In octanol at 183℃; for 0.25h; Heating / reflux; Stage #2: With hydrogenchloride In water Product distribution / selectivity; | 96% |
| Stage #1: polyethylene terephthalate With sodium hydroxide In butan-1-ol at 108℃; for 0.25h; Stage #2: With sulfuric acid pH=2; Product distribution / selectivity; | 96% |
| Stage #1: polyethylene terephthalate With sodium hydroxide In pentan-1-ol at 124℃; for 0.166667h; Stage #2: With sulfuric acid pH=2; Product distribution / selectivity; | 96% |

| Conditions | Yield |
|---|---|
| Stage #1: polyethylene terephthalate With sodium hydroxide In propan-1-ol at 89℃; for 0.25h; Stage #2: With hydrogenchloride In propan-1-ol; water Product distribution / selectivity; | A 96% B n/a |

| Conditions | Yield |
|---|---|
| In 1,2-dimethoxyethane; ethylene glycol | 96% |

| Conditions | Yield |
|---|---|
| With carbon dioxide; oxygen; 1-hydroxy-pyrrolidine-2,5-dione; cobalt(II) acetate In acetic acid at 22℃; under 44734.5 Torr; for 24h; Product distribution / selectivity; | 96% |
-
-
64-19-7
acetic acid

-
-
99-94-5
p-Toluic acid

-
A
-
100-21-0
terephthalic acid

-
B
-
3006-96-0
3-hydroxymethyl-benzoic acid

-
C
-
15561-46-3
p-acetoxymethyl benzoic acid

-
D
-
619-66-9
4-Carboxybenzaldehyde

| Conditions | Yield |
|---|---|
| With N-hydroxyphthalimide; air; cobalt(II) acetate; manganese(II) acetate at 150℃; under 22800 Torr; for 3h; | A 95% B n/a C n/a D n/a |


| Conditions | Yield |
|---|---|
| With C26H30B10Cl2P2Pd In toluene at 60℃; under 760.051 Torr; for 8h; | 95% |
| Stage #1: carbon dioxide With o-phenylenebis(diphenylphosphine); copper(II) acetate monohydrate In 1,4-dioxane at 65℃; for 0.333333h; Schlenk technique; Stage #2: 1.4-dibromobenzene With palladium diacetate; triethylamine; 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene In 1,4-dioxane; toluene at 100℃; for 8h; Schlenk technique; Sealed tube; | 93% |
| Stage #1: carbon dioxide With o-phenylenebis(diphenylphosphine); copper(II) acetate monohydrate In 1,4-dioxane at 65℃; for 0.416667h; Schlenk technique; Stage #2: 1.4-dibromobenzene With palladium diacetate; triethylamine; 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene In 1,4-dioxane; toluene at 100℃; for 20h; Schlenk technique; | 93% |
-
-
119923-98-7
1,4-C6H4(COFp)2

-
A
-
12078-28-3, 38979-86-1
dicarbonylcyclopentadienyliodoiron(II)

-
B
-
100-21-0
terephthalic acid

| Conditions | Yield |
|---|---|
| With iodine In tetrahydrofuran (N2), reaction for 20 min; IR, NMR; | A 77% B 94% |

| Conditions | Yield |
|---|---|
| With bromine In tetrahydrofuran (N2), reaction for 20 min; IR, NMR; | A 85% B 94% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide; amphiphilic resin-supported phosphine-palladium; water at 25℃; under 760 Torr; for 12h; hydroxycarbonylation; | 93% |

| Conditions | Yield |
|---|---|
| Stage #1: (3-amino-4-ethylphenyl)methanol With 2,5-dimethyl-2,5-hexanediol at 67℃; for 2.83333h; Stage #2: With bis(cyclopentadienyl)diphenyltitanium(IV) at 86℃; for 1.5h; Temperature; | 93% |

| Conditions | Yield |
|---|---|
| With oxygen; manganese(II) acetate; cobalt(II) bromide In water; acetic acid at 175 - 220℃; under 11400.8 Torr; for 1h; Product distribution / selectivity; | A 92.4% B n/a |
-
-
558480-54-9
1,3,5-triazin-2,4,6[1H,3H,5H]-trion-1N,3N,5N-tri-O-yl acetate

-
-
100-21-0
terephthalic acid

| Conditions | Yield |
|---|---|
| With nitrogen; acetic acid In titanium; para-xylene | 92% |


| Conditions | Yield |
|---|---|
| With oxygen; manganese (II) acetate tetrahydrate; cobalt(II) diacetate tetrahydrate; 1N,3N,5N-trihydroxy-1,3,5-triazin-2,4,6[1H,3H,5H]-trione In acetic acid at 120℃; under 760.051 Torr; for 15h; | 91% |
| With nitric acid nachfolgend Oxydation mit Permanganat in verduennter Natronlauge; | |
| With sodium hydroxide; chlorine; 1,2,3-trichlorobenzene at 150 - 200℃; anschliessendes Erhitzen mit wss. Natriumhypochlorit-Loesung; |

| Conditions | Yield |
|---|---|
| With Oxone; 2-iodo-3,4,5,6-tetramethylbenzoic acid In water; acetonitrile for 22h; | 91% |
| With Oxone In water; acetonitrile for 19h; Reflux; | 77% |

| Conditions | Yield |
|---|---|
| Stage #1: methanol; terephthalic acid for 0.5h; Reflux; Inert atmosphere; Stage #2: With thionyl chloride for 10h; Reflux; Inert atmosphere; | 100% |
| With thionyl chloride Heating; | 99% |
| Stage #1: methanol; terephthalic acid for 0.5h; Reflux; Stage #2: With thionyl chloride for 12h; Reflux; | 99% |

| Conditions | Yield |
|---|---|
| With thionyl chloride for 2h; Reflux; | 100% |
| With aluminum (III) chloride; Methyltrichlorosilane In tetrachloromethane at 70℃; for 13h; Temperature; Reagent/catalyst; | 99.13% |
| With thionyl chloride for 5h; Reflux; | 98% |
-
-
5122-82-7
1-adamantyl bromomethyl ketone

-
-
100-21-0
terephthalic acid

-
-
123426-29-9
Terephthalic acid bis-(2-adamantan-1-yl-2-oxo-ethyl) ester

| Conditions | Yield |
|---|---|
| With triethylamine In acetone for 6h; Heating; | 100% |
-
-
100-21-0
terephthalic acid

-
-
143-15-7
1-dodecylbromide

-
-
18749-84-3
bis-dodecyl benzene-1,4-dicarboxylate

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In benzene for 18h; Esterification; Heating; | 100% |

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane | 100% |

-
-
100-21-0
terephthalic acid

| Conditions | Yield |
|---|---|
| In water mixing; filtn., solvent evpn.; | 100% |

-
-
100-21-0
terephthalic acid

| Conditions | Yield |
|---|---|
| In water mixing; filtn., solvent evpn.; | 100% |
-
-
100-21-0
terephthalic acid

-
-
17403-09-7
3-(4-piperidinyl)-1H-indole

| Conditions | Yield |
|---|---|
| With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; | 100% |
-
-
100-21-0
terephthalic acid

-
-
46190-30-1
barium terephthalate

| Conditions | Yield |
|---|---|
| With barium(II) chloride dihydrate; potassium hydroxide In water | 100% |
| Stage #1: terephthalic acid With barium(II) nitrate In methanol for 1h; Stage #2: With pyridine for 3h; Reagent/catalyst; Solvent; |

| Conditions | Yield |
|---|---|
| at 170 - 270℃; | 100% |
-
-
100-21-0
terephthalic acid

| Conditions | Yield |
|---|---|
| With 2,6-dimethylpyridine; 1-hydroxy-7-aza-benzotriazole; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane; N,N-dimethyl-formamide at 0 - 25℃; for 4.5h; | 100% |
| Stage #1: terephthalic acid; (3S,4S)-N3,N4-dihexylpyrrolidine-3,4-dicarboxamide hydrochloride With 2,6-dimethylpyridine; 1-hydroxy-7-aza-benzotriazole In dichloromethane; N,N-dimethyl-formamide at 0℃; for 0.0833333h; Stage #2: With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane; N,N-dimethyl-formamide at 0 - 20℃; for 4.5h; | 84% |
-
-
100-21-0
terephthalic acid

| Conditions | Yield |
|---|---|
| In neat (no solvent, solid phase) for 2h; Milling; Inert atmosphere; | 100% |


-
-
100-21-0
terephthalic acid

| Conditions | Yield |
|---|---|
| In neat (no solvent, solid phase) for 2h; Milling; Inert atmosphere; | 100% |


-
-
100-21-0
terephthalic acid

| Conditions | Yield |
|---|---|
| In neat (no solvent, solid phase) for 2h; Milling; Inert atmosphere; | 100% |


| Conditions | Yield |
|---|---|
| at 80℃; for 96 - 168h; | 100% |

-
-
100-21-0
terephthalic acid

-
-
619-81-8, 619-82-9, 1076-97-7
cyclohexane-1,4-dicarboxylic acid

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; 1% Pd/C; hydrogen at 90℃; Reagent/catalyst; Temperature; | 99.9% |
| With hydrogen In water at 200℃; under 30003 Torr; for 3h; Reagent/catalyst; | 98.7% |
| With hydrogen In water at 140℃; under 51716.2 Torr; for 6h; Solvent; Temperature; Autoclave; | 69.8% |

| Conditions | Yield |
|---|---|
| Stage #1: propan-1-ol; terephthalic acid With titanium(IV) isopropylate at 180℃; under 4560.31 Torr; for 3h; Inert atmosphere; Stage #2: propan-1-ol With toluene-4-sulfonic acid at 200℃; under 4560.31 Torr; for 6h; Reagent/catalyst; Inert atmosphere; | 99.6% |

| Conditions | Yield |
|---|---|
| With phenol and titanium tetraisopropoxide resin at 200℃; for 4h; Reagent/catalyst; Dean-Stark; Inert atmosphere; | 99.5% |
| With titanium(IV) isopropylate at 170 - 200℃; Inert atmosphere; Large scale; | 99% |
| With titanium(IV) isopropylate at 170 - 220℃; under 760.051 Torr; for 4.5h; Inert atmosphere; Large scale; | 99% |

| Conditions | Yield |
|---|---|
| With thionyl chloride Heating; | 99% |
| With sulfuric acid | |
| With sulfuric acid Reflux; |
-
-
100-21-0
terephthalic acid

| Conditions | Yield |
|---|---|
| With pyridine; 1-methyl-pyrrolidin-2-one; lithium chloride; triphenyl phosphite at 80℃; | 99% |
-
-
100-21-0
terephthalic acid

-
-
134248-82-1
4,4'‑diamino‑4''‑methoxytriphenylamine

| Conditions | Yield |
|---|---|
| With pyridine; triphenyl phosphite; calcium chloride In 1-methyl-pyrrolidin-2-one at 105℃; for 3h; | 99% |

| Conditions | Yield |
|---|---|
| In toluene byproducts: cyclopentadiene; Ar atmosphere; 20 h at 120°C; ppt. was filtered off, washed with toluene and ether, and dried; elem. anal.; | 99% |

| Conditions | Yield |
|---|---|
| With DMF In solid mixt. of ZnO, C6H4(COOH)2, N2(C2H4)3 (in stoich. ratio 1:1:0.5), (NH4)2SO4 (12% weight fraction of solid reagents) and DMF ground at room temp. for 30 min; detd. by XRD; | 99% |


| Conditions | Yield |
|---|---|
| With DMF In solid mixt. of ZnO, C6H4(COOH)2, N2(C2H4)3 (in stoich. ratio 1:1:0.5), Na2SO4 (12% weight fraction of solid reagents) and DMF ground at room temp. for30 min; detd. by XRD; | 99% |


| Conditions | Yield |
|---|---|
| With DMF In solid mixt. of ZnO, C6H4(COOH)2, N2(C2H4)3 (in stoich. ratio 1:1:0.5), K2SO4 (12% weight fraction of solid reagents) and DMF ground at room temp. for 30 min; detd. by XRD; | 99% |


| Conditions | Yield |
|---|---|
| With triethylamine at 60℃; for 2h; Inert atmosphere; Ionic liquid; | 99% |
-
-
100-21-0
terephthalic acid

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
213275-65-1
[Zn(1,4-benzenedicarboxylate)(H2O)]*DMF

| Conditions | Yield |
|---|---|
| With H2O In neat (no solvent) grinding C6H4(COOH)2 with ZnO and DMF (with H2O content of 10 % v/v) for30 min; detd. by XRD; | 99% |
| In neat (no solvent) grinding C6H4(COOH)2 with ZnO and DMF for 20 min; H. Li et al., J. Am. Chem. Soc. 1998, 120, 8571; detd. by XRD; |

Terephthalic acid Consensus Reports
Terephthalic acid Standards and Recommendations
Terephthalic acid Specification
The Terephthalic acid is an organic compound with the formula C8H6O4. The IUPAC name of this chemical is terephthalic acid. With the CAS registry number 100-21-0, it is also named as 1,4-Benzenedicarboxylic acid. The product's classification codes are Antioxidants; Drug / Therapeutic Agent; Free radical scavengers; Mutation data; Skin / Eye Irritant; TSCA Flag T [Subject to the Section 4 test rule under TSCA]. Besides, it is a white powder, which should be stored in a cool and ventilated place. It is used in the manufacture of synthetic resin, synthetic fibers, plasticizers and chromatography reagents.
Physical properties about Terephthalic acid are: (1)ACD/LogP: 2.00; (2)ACD/BCF (pH 5.5): 1; (3)ACD/BCF (pH 7.4): 1; (4)ACD/KOC (pH 5.5): 1; (5)ACD/KOC (pH 7.4): 1; (6)#H bond acceptors: 4; (7)#H bond donors: 2; (8)#Freely Rotating Bonds: 2; (9)Polar Surface Area: 74.6 Å2; (10)Index of Refraction: 1.618; (11)Molar Refractivity: 40.113 cm3; (12)Molar Volume: 114.493 cm3; (13)Polarizability: 15.902×10-24cm3; (14)Surface Tension: 70.304 dyne/cm; (15)Density: 1.451 g/cm3; (16)Flash Point: 205.267 °C; (17)Enthalpy of Vaporization: 67.722 kJ/mol; (18)Boiling Point: 392.373 °C at 760 mmHg.
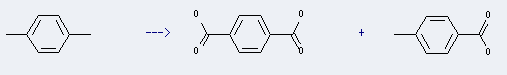
Preparation: this chemical can be prepared by 1,4-dimethyl-benzene. This reaction will need reagent NHPI, Co(acac)2, O2 and solvent acetic acid. The reaction time is 12 hours with reaction temperature of 100 °C. The yield is about 23 %.
Uses of Terephthalic acid: it can be used to produce N,N'-p-phenylene-bis-carbamic acid diethyl ester by heating. It will need reagent polymer-supported diphenylphosphoryl azide, Et3N and solvent benzene with reaction time of 24 hours. The yield is about 36%.
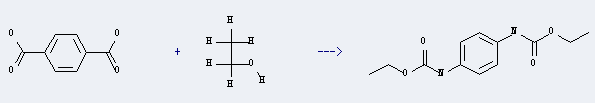
When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes, respiratory system and skin. When you are using it, wear suitable protective clothing. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: c1cc(ccc1C(=O)O)C(=O)O
(2)InChI: InChI=1/C8H6O4/c9-7(10)5-1-2-6(4-3-5)8(11)12/h1-4H,(H,9,10)(H,11,12)
(3)InChIKey: KKEYFWRCBNTPAC-UHFFFAOYAF
(4)Std. InChI: InChI=1S/C8H6O4/c9-7(10)5-1-2-6(4-3-5)8(11)12/h1-4H,(H,9,10)(H,11,12)
(5)Std. InChIKey: KKEYFWRCBNTPAC-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LDLo | intravenous | 767mg/kg (767mg/kg) | Toxicology and Applied Pharmacology. Vol. 18, Pg. 469, 1971. | |
| mouse | LD50 | intraperitoneal | 1430mg/kg (1430mg/kg) | Chemical and Pharmaceutical Bulletin. Vol. 16, Pg. 1655, 1968. | |
| mouse | LD50 | oral | 3200mg/kg (3200mg/kg) | Kodak Company Reports. Vol. 21MAY1971, | |
| rat | LD50 | oral | > 6400mg/kg (6400mg/kg) | Kodak Company Reports. Vol. 21MAY1971, |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View