-
Name
Triphenylene
- EINECS 205-922-9
- CAS No. 217-59-4
- Article Data201
- CAS DataBase
- Density 1.19 g/cm3
- Solubility 6.6ug/L(25.00 oC)
- Melting Point 195-198 °C(lit.)
- Formula C18H12
- Boiling Point 424.998 °C at 760 mmHg
- Molecular Weight 228.293
- Flash Point 209.141 °C
- Transport Information UN 3077 9/PG 3
- Appearance white to beige crystalline needles
- Safety 22-24/25-61-60-39-26
- Risk Codes 41
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi, N
N
- Synonyms 1,2,3,4-Dibenznaphthalene;9,10-Benzophenanthrene;9,10-Benzphenanthrene;Benzo[l]phenanthrene;Isochrysene;NSC 57455;
- PSA 0.00000
- LogP 5.14620
Synthetic route

| Conditions | Yield |
|---|---|
| With {Co(II)(DBF2)2(H2O)2} In acetonitrile at 20℃; for 18h; Catalytic behavior; Time; Sealed tube; UV-irradiation; Inert atmosphere; | 100% |
| With trifluorormethanesulfonic acid; 5% Pd/Al2O3; oxygen In dichloromethane at 20℃; for 1.4h; | 96% |
| With iodine In benzene for 168h; UV-irradiation; | 63.9% |
-
-
75295-57-7
2-(2-bromophenyl)biphenyl

-
-
217-59-4
triphenylene

| Conditions | Yield |
|---|---|
| With bis(benzonitrile)palladium(II) chloride; caesium carbonate; tris<3,5-bis(trifluoromethyl)phenyl>phosphane In toluene at 110℃; for 24h; | 100% |

| Conditions | Yield |
|---|---|
| With aluminum oxide at 250℃; for 0.166667h; Inert atmosphere; | 99.5% |
| With dimethyldimesitylsilane In chlorobenzene at 110℃; for 8h; Friedel Crafts reaction; Inert atmosphere; | 97% |

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon at 300℃; for 10h; Inert atmosphere; | 99% |
| palladium on activated charcoal at 310℃; for 25h; | 82% |
| 5% Pd(II)/C(eggshell) In Exxsol D110 at 225℃; for 40h; Product distribution / selectivity; Industry scale; | 70% |

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; potassium carbonate; p-benzoquinone In dimethyl sulfoxide at 140℃; for 4h; Schlenk technique; Inert atmosphere; | 99% |

-
-
217-59-4
triphenylene

| Conditions | Yield |
|---|---|
| Stage #1: 1-(biphenyl-2-yl)cyclopent-3-ene-1-carbaldehyde With trifluorormethanesulfonic acid In dichloromethane at 0℃; for 3h; Stage #2: With 2,3-dicyano-5,6-dichloro-p-benzoquinone In toluene for 3h; Reflux; | 97% |
-
-
1338363-01-1
[1,1':2',1"-terphenyl]-2-yl trifluoromethanesulfonate

-
-
217-59-4
triphenylene

| Conditions | Yield |
|---|---|
| With potassium carbonate; palladium dichloride; tris[tert-butyl]phosphonium tetrafluoroborate; Trimethylacetic acid at 140℃; for 20h; Schlenk technique; Inert atmosphere; | 96% |
-
-
17296-31-0
2-chloro-1,1':2',1''-terphenyl

-
-
217-59-4
triphenylene

| Conditions | Yield |
|---|---|
| With sodium carbonate In water; acetone for 48h; UV-irradiation; Inert atmosphere; Sealed tube; regioselective reaction; | 95% |

| Conditions | Yield |
|---|---|
| With n-butyllithium In hexane at -78 - 25℃; | 93% |

| Conditions | Yield |
|---|---|
| With dicyclohexyl-(2',6'-dimethoxybiphenyl-2-yl)-phosphane; tris-(dibenzylideneacetone)dipalladium(0); potassium carbonate In 1,2-dimethoxyethane at 135℃; for 24h; Catalytic behavior; Reagent/catalyst; Schlenk technique; Inert atmosphere; | A 92% B 10% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-bromo-1-chlorobenzene With tert.-butyl lithium; manganese(ll) chloride In tetrahydrofuran; pentane at -78℃; for 0.333333h; Inert atmosphere; Schlenk technique; Stage #2: In tetrahydrofuran; pentane at 25℃; for 1h; Inert atmosphere; Schlenk technique; | 91% |
| With n-butyllithium; diethyl ether at -60℃; anschliessendes Erwaermen; |
-
-
1276113-19-9
2-(trimethylsilyl)phenyl 1,1,2,2,3,3,4,4,4-nonafluorobutane-1-sulfonate

-
-
217-59-4
triphenylene

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); cesium fluoride In acetonitrile at 55℃; for 16h; Microwave irradiation; | 90% |

-
A
-
217-59-4
triphenylene

| Conditions | Yield |
|---|---|
| With C18H19Cl2N2PPd; sodium acetate In N,N-dimethyl-formamide at 160℃; for 0.333333h; Inert atmosphere; Schlenk technique; | A 8% B 90% |
-
-
88284-48-4
2-(trimethylsilyl)phenyl trifluoromethanesulfonate

-
-
217-59-4
triphenylene

| Conditions | Yield |
|---|---|
| With (triphenylphosphine)gold(I) chloride; cesium fluoride In acetonitrile at 60℃; for 6h; Catalytic behavior; Reagent/catalyst; Solvent; Temperature; Inert atmosphere; | 88% |
| With sodium amalgam; tetrakis(triphenylphosphine) palladium(0); 18-crown-6 ether; cesium fluoride In tetrahydrofuran at 20℃; for 16h; Catalytic behavior; Inert atmosphere; | 87% |
| With C23H20Br2N4O4Pd; cesium fluoride In acetonitrile at 60℃; for 2h; Reagent/catalyst; Temperature; Inert atmosphere; | 85% |

| Conditions | Yield |
|---|---|
| With bis(tri-t-butylphosphine)palladium(0) In tetrahydrofuran at 60℃; for 12h; | 87% |
-
-
88284-48-4
2-(trimethylsilyl)phenyl trifluoromethanesulfonate

-
-
762-42-5
dimethyl acetylenedicarboxylate

-
A
-
217-59-4
triphenylene

-
B
-
15810-16-9
dimethyl 9,10-phenanthrenedicarboxylic acid

-
C
-
36063-07-7
tetramethyl naphthalene-1,2,3,4-tetracarboxylate

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); cesium fluoride In acetonitrile Ambient temperature; | A 2% B 84% C 7% |
| With cesium fluoride; tetrakis(triphenylphosphine) palladium(0) In acetonitrile at 20℃; for 13h; Product distribution; Further Variations:; Catalysts; cocyclization; | A 2% B 84% C 7% |
| With cesium fluoride; tetrakis(triphenylphosphine) palladium(0) In acetonitrile at 20℃; for 12h; cocyclization; | A 2% B 84% C 7% |

-
-
88284-48-4
2-(trimethylsilyl)phenyl trifluoromethanesulfonate

-
-
536-74-3
phenylacetylene

-
-
217-59-4
triphenylene

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0) In acetonitrile at 20℃; for 12h; Inert atmosphere; | 83% |
-
-
625-86-5
2,5-dimethylfuran

-
-
7579-74-0
1-(tri-n-butylstannyl)-2-fluorobenzene

-
A
-
217-59-4
triphenylene

-
B
-
4705-93-5
1,4-dihydro-1,4-dimethyl-1,4-epoxynaphthalene

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran; hexane at -78 - 25℃; | A 83% B 17% |
| With n-butyllithium In hexane at -78 - 25℃; | A 28% B 72% |


| Conditions | Yield |
|---|---|
| With (bis(tricyclohexyl)phosphine)palladium(II) dichloride; P(p-CH3OC6H4)3; caesium carbonate In 1,4-dioxane for 24h; Suzuki-Miyaura Coupling; Inert atmosphere; Reflux; regioselective reaction; | 83% |
-
-
269410-07-3
1,2-bis(4,4,5,5-tetramethyl-[1,3,2]dioxabororan-2-yl)benzene

-
-
13029-09-9
2,2'-dibromobiphenyl

-
-
217-59-4
triphenylene

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); potassium carbonate In tetrahydrofuran; water at 60℃; for 48h; Inert atmosphere; | 82% |

| Conditions | Yield |
|---|---|
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In toluene at 110℃; for 4h; Inert atmosphere; | 81% |
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone Inert atmosphere; | 8% |
| With selenium at 320℃; |

| Conditions | Yield |
|---|---|
| With bis(benzonitrile)palladium(II) dichloride; caesium carbonate; tris<3,5-bis(trifluoromethyl)phenyl>phosphane In toluene at 110℃; for 24h; Reagent/catalyst; Inert atmosphere; Schlenk technique; | 81% |
| With bis(benzonitrile)palladium(II) chloride; caesium carbonate; tris<3,5-bis(trifluoromethyl)phenyl>phosphane In toluene at 110℃; for 24h; Catalytic behavior; Mechanism; Time; Reagent/catalyst; Solvent; Temperature; Inert atmosphere; Schlenk technique; | 81% |
-
-
189999-35-7
[1,1'-biphenyl]-2,2'-iodonium trifluoromethanesulfonate

-
-
65-85-0
benzoic acid

-
-
217-59-4
triphenylene

| Conditions | Yield |
|---|---|
| With potassium phosphate; palladium diacetate In N,N-dimethyl-formamide at 145℃; for 16h; Catalytic behavior; Reagent/catalyst; Solvent; Temperature; Concentration; | 81% |
| With palladium diacetate; potassium carbonate In N,N-dimethyl-formamide at 145℃; for 15h; | 30% |
| With palladium diacetate; potassium carbonate In 1-methyl-pyrrolidin-2-one at 110℃; for 17h; | 15% |
| With silver hexafluoroantimonate; palladium(II) trifluoroacetate; trifluoroacetic acid at 130℃; for 24h; Molecular sieve; Sealed tube; | 6% |
-
-
189999-35-7
[1,1'-biphenyl]-2,2'-iodonium trifluoromethanesulfonate

-
-
118-91-2
ortho-chlorobenzoic acid

-
-
217-59-4
triphenylene

| Conditions | Yield |
|---|---|
| With palladium diacetate; potassium carbonate In 1-methyl-pyrrolidin-2-one at 110℃; for 17h; Catalytic behavior; Reagent/catalyst; Solvent; Temperature; | 81% |
| With palladium diacetate; potassium carbonate In 1-methyl-pyrrolidin-2-one at 110℃; for 17h; | 80% |
-
-
1437769-72-6
2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl trifluoromethanesulfonate

-
-
217-59-4
triphenylene

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; bis[2-(diphenylphosphino)phenyl] ether; bis(dibenzylideneacetone)-palladium(0) In toluene at 100℃; Schlenk technique; Inert atmosphere; | 80% |

-
-
88284-48-4
2-(trimethylsilyl)phenyl trifluoromethanesulfonate

-
-
217-59-4
triphenylene

| Conditions | Yield |
|---|---|
| With sodium amalgam; tetrakis(triphenylphosphine) palladium(0); 18-crown-6 ether; cesium fluoride In tetrahydrofuran at 20℃; for 16h; Catalytic behavior; Inert atmosphere; | 80% |
-
-
205178-58-1
naphthalen-1-yl-2-iodobenzoate

-
A
-
217-59-4
triphenylene

-
B
-
55377-35-0
6H-benzo[d]naphtho[1,2-b]pyran-6-one

| Conditions | Yield |
|---|---|
| With C18H19Cl2N2PPd; sodium acetate In N,N-dimethyl-formamide at 160℃; for 0.333333h; Inert atmosphere; Schlenk technique; | A 7% B 79% |

-
-
88284-48-4
2-(trimethylsilyl)phenyl trifluoromethanesulfonate

-
A
-
217-59-4
triphenylene

| Conditions | Yield |
|---|---|
| With sodium amalgam; tetrakis(triphenylphosphine) palladium(0); 18-crown-6 ether; cesium fluoride In tetrahydrofuran at 20℃; for 16h; Catalytic behavior; Reagent/catalyst; Schlenk technique; Inert atmosphere; | A 67% B 79% |


| Conditions | Yield |
|---|---|
| With hydrazine hydrate; palladium on activated charcoal In ethanol for 0.5h; Heating; | A 4% B 78% |
-
-
269410-06-2
2-(2-bromophenyl)-4,4,5,5-tetramethyl-[1,3,2]dioxaborolane

-
-
217-59-4
triphenylene

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; bis[2-(diphenylphosphino)phenyl] ether; bis(dibenzylideneacetone)-palladium(0) In toluene at 100℃; for 16h; Catalytic behavior; Reagent/catalyst; Solvent; Temperature; Time; Schlenk technique; Inert atmosphere; Sealed tube; | 78% |

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride In dichloromethane at 0 - 20℃; Friedel-Crafts Acylation; Inert atmosphere; | 100% |
| With aluminium trichloride In nitrobenzene for 12h; Ambient temperature; | 92% |
| With aluminium trichloride In nitrobenzene at 0 - 22℃; for 20h; | 81% |
-
-
217-59-4
triphenylene

-
-
17777-56-9
triphenylene-d12

| Conditions | Yield |
|---|---|
| With [mesitylenium]B(C6F5)4; benzene-d6 at 20℃; for 24h; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With zirconium(IV) chloride In dichloromethane at 20℃; for 64h; Friedel-Crafts Acylation; regioselective reaction; | 97% |
-
-
217-59-4
triphenylene

-
-
82632-80-2
2,3,6,7,10,11-hexabromo-triphenylene

| Conditions | Yield |
|---|---|
| With bromine; iron In nitrobenzene at 20 - 205℃; for 18.25h; | 95% |
| With bromine; iron In nitrobenzene at 20 - 205℃; for 18.25h; | 95% |
| With bromine; iron In nitrobenzene at 20 - 210℃; for 3.33h; Inert atmosphere; | 94.8% |
-
-
42333-78-8
2,4,6-tri(4-pyridyl)-1,3,5-triazine

-
-
217-59-4
triphenylene

-
-
2923-28-6
silver trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| Stage #1: [Cp*2Rh2(2,2'-bisbenzimidazole)Cl2]; silver trifluoromethanesulfonate at 20℃; for 12h; Darkness; Inert atmosphere; Schlenk technique; Stage #2: 2,4,6-tri(4-pyridyl)-1,3,5-triazine for 4h; Inert atmosphere; Schlenk technique; Stage #3: triphenylene | 92.5% |

-
-
42333-78-8
2,4,6-tri(4-pyridyl)-1,3,5-triazine

-
-
217-59-4
triphenylene

-
-
63994-76-3
(ethylenediamine)palladium(II) dinitrate

-
-
7732-18-5
water

| Conditions | Yield |
|---|---|
| In water-d2 at 40℃; for 2h; | 92% |

-
-
217-59-4
triphenylene

-
B
-
82632-80-2
2,3,6,7,10,11-hexabromo-triphenylene

| Conditions | Yield |
|---|---|
| With bromine; iron In nitrobenzene at 205℃; for 12h; | A n/a B 92% |
-
-
42333-78-8
2,4,6-tri(4-pyridyl)-1,3,5-triazine

-
-
217-59-4
triphenylene

-
-
63994-76-3
(ethylenediamine)palladium(II) dinitrate

-
-
7732-18-5
water

| Conditions | Yield |
|---|---|
| In water-d2 at 40℃; for 23h; | 91% |


-
-
217-59-4
triphenylene

-
-
42516-72-3
(η6-1,3,5-cyclooctatriene)(η4-1,5-cyclooctadiene)ruthenium(0)

-
-
942293-23-4
[Ru(η6-triphenylene)(1-5-η5-cyclooctadienyl)]PF6

| Conditions | Yield |
|---|---|
| In diethyl ether under N2 atm. to soln. Ru complex and triphenylene in Et2O HPF6 was added; ppt. was recrystd. from CH2Cl2-Et2O at -30°C; elem. anal.; | 90% |

-
-
42333-76-6
2,4,6-tris(pyridin-3-yl)-1,3,5-triazine

-
-
217-59-4
triphenylene

-
-
2923-28-6
silver trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| In methanol byproducts: AgCl; stirred at reflux for 24 h; filtered, solvent-removed, dissolved in CH2Cl2, pptd. (Et2O), filtered, dried (vac.); elem. anal.; | 89% |


| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 8h; In air; | 88% |
| In methanol react. tetrol and 1,4-benzenedi(boronic acid), triphenylene in MeOH at room temp. for 6 h; | 86% |

-
-
217-59-4
triphenylene

-
-
1313497-83-4
3,6‐di‐tert‐butyl‐1,8‐diethynylcarbazole

| Conditions | Yield |
|---|---|
| Stage #1: 3,6‐di‐tert‐butyl‐1,8‐diethynylcarbazole With sodium hydroxide In methanol at 80℃; for 1h; Stage #2: triphenylene; C42H58Au2I2N4 In methanol at 80℃; for 4h; | 88% |

-
-
42333-78-8
2,4,6-tri(4-pyridyl)-1,3,5-triazine

-
-
217-59-4
triphenylene


-
-
2923-28-6
silver trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| In methanol to mixt. of Ru complex and Ag triflate in MeOH added triazine deriv. andtriphenylene; mixt. stirred at reflux for 24 h; filtered, solvent removed, residue dissolved in CH2Cl2, Et2O added for pptn.. solid filtered, dried under vac.; elem. anal.; | 87% |

-
-
217-59-4
triphenylene

-
-
19111-87-6
2-bromobenzo[9,10]phenanthrene

| Conditions | Yield |
|---|---|
| With bromine In dichloromethane at 20℃; for 12h; Inert atmosphere; | 86.3% |
| With N-Bromosuccinimide; iron(III) chloride hexahydrate In tetrachloromethane for 7h; Reflux; Heating; | 54% |
| With carbon disulfide; bromine; iron |
-
-
42333-78-8
2,4,6-tri(4-pyridyl)-1,3,5-triazine

-
-
217-59-4
triphenylene

-
-
2923-28-6
silver trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| In dichloromethane Ru compd. (0.14 mmol) and AgOTf (0.28 mmol) stirred at room temp. for 3 h, filtered, tpt (0.09 mmol) and triphenylene (0.04 mmol) added, mixt. refluxed for 15 h; evapd., dissolved (CH2Cl2), pptd. (Et2O), filtered off, dried (vac.), elem. anal.; | 86% |

Triphenylene Consensus Reports
Triphenylene Specification
Triphenylene is an organic compound with the formula C18H12, and its systematic name is the same with the product name. With the CAS registry number 217-59-4, it is also named as 9,10-Benzphenanthrene. It belongs to the product categories of Aromatic Hydrocarbons (substituted) & Derivatives; Naphthyridine,Quinoline. Its EINECS number is 205-922-9. In addition, the molecular weight is 228.29. Its classification codes are: (1)Mutation data; (2)Tumor data. It may have uses in optics and electronics. Triphenylene can be isolated from coal tar, but it is also made synthetically using benzyne chemistry, as it is a trimer of benzyne.
Physical properties of Triphenylene are: (1)ACD/LogP: 5.729; (2)# of Rule of 5 Violations: 1; (3)ACD/LogD (pH 5.5): 5.73; (4)ACD/LogD (pH 7.4): 5.73; (5)ACD/BCF (pH 5.5): 13312.29; (6)ACD/BCF (pH 7.4): 13312.29; (7)ACD/KOC (pH 5.5): 31169.38; (8)ACD/KOC (pH 7.4): 31169.38; (9)#H bond acceptors: 0; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 0; (12)Index of Refraction: 1.771; (13)Molar Refractivity: 79.78 cm3; (14)Molar Volume: 191.781 cm3; (15)Polarizability: 31.627×10-24cm3; (16)Surface Tension: 53.54 dyne/cm; (17)Density: 1.19 g/cm3; (18)Flash Point: 209.141 °C; (19)Enthalpy of Vaporization: 65.327 kJ/mol; (20)Boiling Point: 424.998 °C at 760 mmHg; (21)Vapour Pressure: 0 mmHg at 25°C.
Preparation: this chemical can be prepared by 1,2,3,4,5,6,7,8,9,10,11,12-dodecahydro-triphenylene at the temperature of 310 °C. This reaction time is 25 hours. This reaction will also need catalyst Pd/C. The yield is about 82%.
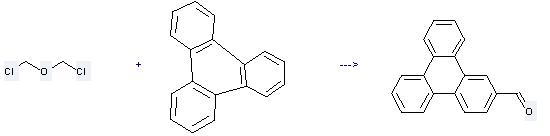
Uses of Triphenylene: it can be used to produce triphenylene-2-carbaldehyde at the ambient temperature. It will need reagent TiCl4 and solvent CH2Cl2 with the reaction time of 3 hours. The yield is about 78%.
When you are using this chemical, please be cautious about it as the following:
This chemical has a risk of serious damage to eyes. You should not breathe dust. When using it, you must avoid contact with skin and eyes. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need wear eye/face protection. This material and its container must be disposed of as hazardous waste. You should avoid releasing it to the environment, and you need to refer to special instructions/safety data sheet.
You can still convert the following datas into molecular structure:
(1)SMILES: c1ccc2c(c1)c3ccccc3c4c2cccc4
(2)Std. InChI: InChI=1S/C18H12/c1-2-8-14-13(7-1)15-9-3-4-11-17(15)18-12-6-5-10-16(14)18/h1-12H
(3)Std. InChIKey: SLGBZMMZGDRARJ-UHFFFAOYSA-N
Related Products
- Triphenylene
- Triphenylene, 2-nitro-
- Triphenylene-D12
- 2176-45-6
- 21764-56-7
- 217650-27-6
- 217661-27-3
- 2176-62-7
- 2176-98-9
- 21770-81-0
- 21771-88-0
- 21774-03-8
- 2177-47-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View