-
Name
Triphenylethylene
- EINECS 200-395-1
- CAS No. 58-72-0
- Article Data274
- CAS DataBase
- Density 1.072 g/cm3
- Solubility
- Melting Point 68-71 °C
- Formula C20H16
- Boiling Point 358.2 °C at 760 mmHg
- Molecular Weight 256.347
- Flash Point 165 °C
- Transport Information
- Appearance White to slightly beige powder
- Safety 26-36/37-60-61
- Risk Codes 22-36-50/53
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, N
N
- Synonyms Ethylene,triphenyl- (6CI,8CI);1,1,2-Triphenylethene;1,1,2-Triphenylethylene;Benzilidenediphenylmethane;NSC 17535;Triphenylethene;Triphenylethylene;
- PSA 0.00000
- LogP 5.27550
Synthetic route

| Conditions | Yield |
|---|---|
| With water; acetic acid; bis-triphenylphosphine-palladium(II) chloride at 20℃; for 6h; | 100% |
| With bis-triphenylphosphine-palladium(II) chloride; water; acetic acid at 20℃; for 6h; Catalytic behavior; Reagent/catalyst; Temperature; Inert atmosphere; Sealed tube; | 94% |
-
-
591-50-4
iodobenzene

-
-
173603-23-1
(E)-2,2′-(1-phenylethene-1,2-diyl)bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane)

-
-
58-72-0
Triphenylethylene

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); sodium hydroxide In tetrahydrofuran; water at 70℃; for 4h; Suzuki-Miyaura Coupling; | 100% |
-
-
384360-11-6
1-dimethyl(2-pyridyl)silyl-1,2,3-triphenylethene

-
-
58-72-0
Triphenylethylene

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran at 60℃; for 1h; | 99% |

| Conditions | Yield |
|---|---|
| With tetraethylammonium perchlorate; triethylamine In ethanol; dimethyl sulfoxide at 20℃; for 18h; Electrolysis; Green chemistry; | 98% |
| With lithium aluminium tetrahydride; nickel dichloride In tetrahydrofuran < 0 deg C; | 95% |
| With lithium aluminium tetrahydride; nickel dichloride In tetrahydrofuran Product distribution; < 0 deg C; other triarylvinyl halogenides, other transition metal halides; | 95% |
| With 2,3-diethynylquinoxaline; tetrabutylammonium tetrafluoroborate In N,N-dimethyl-formamide at 25℃; Product distribution; Rate constant; Thermodynamic data; -1.2 V vs. Ag/AgI; various mediators; indirect electrochemical reduction of vinyl halides and related compounds; rate constants and free energies of activation for electron transfer from electrochemically generated anion radicals to vinyl halides; | |
| With Diethyl phosphonate; tert-butyl alcohol In acetonitrile at 25℃; Product distribution; Kinetics; Further Variations:; Solvents; |

| Conditions | Yield |
|---|---|
| Stage #1: phenylmagnesium bromide; diphenyl acetylene With nickel(II) chloride hexahydrate In tetrahydrofuran; toluene at 20℃; for 1h; Inert atmosphere; Schlenk technique; Stage #2: With water In tetrahydrofuran; toluene Catalytic behavior; Reagent/catalyst; Solvent; Concentration; Inert atmosphere; Schlenk technique; | 98% |
| With manganese(ll) chloride In tetrahydrofuran; toluene at 100℃; for 4h; | 60% |
| Stage #1: phenylmagnesium bromide; diphenyl acetylene With 1,1'-bis-(diphenylphosphino)ferrocene; iron(III)-acetylacetonate; 1,2-dichloro-2-methylpropane In tetrahydrofuran at 0℃; for 1h; Inert atmosphere; Schlenk technique; Stage #2: With hydrogenchloride In tetrahydrofuran; water Catalytic behavior; Reagent/catalyst; | 24 %Chromat. |

| Conditions | Yield |
|---|---|
| With silver(I) acetate; palladium diacetate; acetic acid at 110℃; for 6h; Heck reaction; | 97% |
| With dipalladium(II)(1,1'-di-t-butyl-3,3'-(1,2-ethanediyl)bisimidazolium)dipyridinetetradichloride; tetrabutylammomium bromide; sodium acetate In N,N-dimethyl acetamide at 120℃; for 18h; Heck Reaction; Inert atmosphere; | 71% |

| Conditions | Yield |
|---|---|
| With 15-crown-5; sodium hydride In tetrahydrofuran at 0 - 25℃; for 3h; | 96% |
| With potassium tert-butylate; toluene |

| Conditions | Yield |
|---|---|
| With triethylamine; bis(acetato)bis(triphenylphosphine)palladium(0) In acetonitrile at 80℃; for 3.5h; | 96% |
| With tris-(dibenzylideneacetone)dipalladium(0); potassium carbonate; chlorobenzene In ethanol at 70℃; for 2h; Catalytic behavior; Reagent/catalyst; Inert atmosphere; Schlenk technique; | 80% |
-
-
22021-09-6
1,2,2-triphenyl-1-iodoethylene

-
A
-
58-72-0
Triphenylethylene

-
B
-
1836-87-9
9-Benzylidene-9H-fluorene

| Conditions | Yield |
|---|---|
| With palladium diacetate; cesium pivalate; bis-diphenylphosphinomethane In N,N-dimethyl-formamide at 100℃; for 24h; | A 4% B 96% |
| With sodium acetate; dimethyl amine; bis-diphenylphosphinomethane; palladium diacetate at 100℃; for 36h; | A 48% B 52% |

| Conditions | Yield |
|---|---|
| With (N,N-diisopropylamino)diphenylphosphine; potassium carbonate; palladium dichloride In tetrahydrofuran at 20 - 65℃; Inert atmosphere; | 96% |
| With acetic acid; tetrakis(triphenylphosphine) palladium(0) In 1,4-dioxane at 80℃; for 12h; | 91% |
| With water; palladium diacetate; bis(pinacol)diborane; tricyclohexylphosphine In tetrahydrofuran at 80℃; for 4h; | 90% |

| Conditions | Yield |
|---|---|
| With potassium fluoride; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; palladium diacetate; propionic acid at 20℃; for 1h; sealed tube; | 95% |
| With palladium diacetate; sodium acetate In acetic acid at 25℃; for 27h; | 69 % Chromat. |
-
-
108-86-1
bromobenzene

-
-
501-65-5
diphenyl acetylene

-
-
73183-34-3
bis(pinacol)diborane

-
A
-
58-72-0
Triphenylethylene

-
B
-
632-51-9
1,1,2,2-tetraphenylethylene

| Conditions | Yield |
|---|---|
| Stage #1: diphenyl acetylene; bis(pinacol)diborane With tetrakis(triphenylphosphine)platinum In 1,4-dioxane at 180℃; for 0.5h; microwave irradiation; Stage #2: bromobenzene With palladium diacetate; potassium hydroxide; triphenylphosphine In 1,4-dioxane at 140℃; for 0.5h; Suzuki cross-coupling reaction; microwave irradiation; Further stages.; | A n/a B 95% |

-
-
4406-72-8
2-phenyl[1,3,2]dioxaborolane

-
-
24892-82-8
1-bromo-2-(1-phenylpropen-1-yl)benzene

-
-
58-72-0
Triphenylethylene

| Conditions | Yield |
|---|---|
| With tris(o-methoxyphenyl)phosphine; palladium diacetate; palladium; cesium pivalate In tetrahydrofuran at 110℃; for 3h; Schlenk technique; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| With [1,1'-bis(diphenylphosphino)ferrocene]nickel(II) chloride; ethanol; potassium hexamethylsilazane In toluene at 150℃; for 8h; Inert atmosphere; Molecular sieve; | 95% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide; triphenylphosphine; palladium diacetate In tetrahydrofuran; methanol at 25℃; for 1h; Suzuki cross-coupling; | 94% |

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); trifuran-2-yl-phosphane; lithium tert-butoxide In toluene at 80℃; for 3h; Inert atmosphere; | 94% |

| Conditions | Yield |
|---|---|
| With palladium(II) acetylacetonate; N,N,N,N,-tetramethylethylenediamine; copper(I) bromide In 1-methyl-pyrrolidin-2-one at 170℃; for 16h; Inert atmosphere; | 94% |

| Conditions | Yield |
|---|---|
| iodine at 60℃; for 4h; | 93% |
| With potassium hydrogensulfate at 155 - 160℃; | |
| With acetic acid |

| Conditions | Yield |
|---|---|
| With 1,1'-bis-(diphenylphosphino)ferrocene; cesium acetate; copper diacetate In tetrahydrofuran at 80℃; for 24h; Reagent/catalyst; diastereoselective reaction; | 93% |
| With formic acid; triethylamine; bis(acetato)bis(triphenylphosphine)palladium(0) In acetonitrile at 80℃; for 6.5h; Product distribution; Mechanism; reaction with various aryl iodides; | 87% |
| With formic acid; triethylamine; bis(acetato)bis(triphenylphosphine)palladium(0) In acetonitrile at 80℃; for 6.5h; | 87% |
| Stage #1: diphenyl acetylene With ethylmagnesium bromide; iron(II) chloride In diethyl ether at 20℃; for 0.25h; Inert atmosphere; Stage #2: iodobenzene With bis(triphenylphosphine)nickel(II) chloride In diethyl ether at 20℃; for 3.5h; Inert atmosphere; Stage #3: With hydrogenchloride; water In diethyl ether | 80% |
| With sodium; triphenylphosphine; palladium diacetate In methanol at 25℃; for 24h; | 64% |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 120℃; for 24h; Heck Reaction; Inert atmosphere; | 93% |
| With silver(I) acetate; palladium diacetate; acetic acid at 110℃; for 0.5h; Heck reaction; | 92% |
| With silver(I) acetate; palladium diacetate In acetic acid at 110℃; for 2h; | 88% |

| Conditions | Yield |
|---|---|
| With hafnium(IV) trifluoromethanesulfonate; 1-n-butyl-3-methylimidazolium hexafluoroantimonate at 85℃; for 1h; Friedel-Crafts alkenylation; | 92% |
| With 1-n-butyl-3-methylimidazolium hexafluoroantimonate; hafnium(IV) trifluoromethanesulfonate at 85℃; for 1h; Friedel-Crafts alkenylation; | 92% |
| With tributyl borane; Pd2(p-Tol)2(μ-OH)(μ-dpfam) In tetrahydrofuran at 120℃; for 17h; | 64% |

| Conditions | Yield |
|---|---|
| Stage #1: phenyl trimethylsiloxane; diphenyl acetylene With chloro(1,5-cyclooctadiene)rhodium(I) dimer; tetrabutyl ammonium fluoride; copper diacetate; triphenylphosphine In toluene at 20℃; for 2h; Stage #2: With water In toluene at 110℃; for 12h; | 92% |

| Conditions | Yield |
|---|---|
| With palladium(II) acetylacetonate; N,N,N,N,-tetramethylethylenediamine; copper(I) bromide at 140℃; for 16h; Inert atmosphere; | 92% |

| Conditions | Yield |
|---|---|
| In chloroform-d1 for 2h; Photolysis; Title compound not separated from byproducts; | A 9% B 91% |
-
-
1607-57-4
Triphenylvinyl bromide

-
-
2633-75-2
ethylzinc chloride

-
A
-
58-72-0
Triphenylethylene

-
B
-
63019-13-6
but-1-ene-1,1,2-triyltribenzene

| Conditions | Yield |
|---|---|
| With 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; palladium diacetate at 100℃; Product distribution; Further Variations:; Reagents; Catalysts; Temperatures; Negishi coupling; | A n/a B 91% |


| Conditions | Yield |
|---|---|
| acetylacetonatodicarbonylrhodium(l) In water; toluene at 110℃; for 12h; Product distribution; Further Variations:; Catalysts; Solvents; | 90% |

| Conditions | Yield |
|---|---|
| With palladium diacetate; trifluoroacetic acid; naphthalene-1,8-diamine In 1,4-dioxane; water at 120℃; for 24h; Catalytic behavior; Reagent/catalyst; Solvent; Inert atmosphere; Sealed tube; | 90% |

| Conditions | Yield |
|---|---|
| With [bis(acetato)-(η-p-cymene)-ruthenium] In 1,4-dioxane; n-heptane; 1,3,5-trimethyl-benzene at 80℃; for 48h; Inert atmosphere; Sealed tube; regioselective reaction; | 90% |
| With α-picoline; [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; guanidinium carbonate; acetic acid In toluene at 120℃; Inert atmosphere; | 62% |
| With [Ru(O2CMes)2(p-cymene)]; vanadia In toluene at 100℃; for 24h; Inert atmosphere; | 43% |

| Conditions | Yield |
|---|---|
| With palladium diacetate; potassium carbonate; tris-(o-tolyl)phosphine In N,N-dimethyl-formamide at 130℃; for 48h; Heck reaction; Inert atmosphere; | 89% |
| With C24H26N4O2Pd2*2ClO4(1-); sodium acetate In 1-methyl-pyrrolidin-2-one at 140℃; Sealed tube; Air; | 61% |
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 150℃; for 48h; Heck Reaction; Inert atmosphere; | 24% |
| With {2,6-bis[(di-1-piperidinylphosphino)amino]phenyl}palladium(II) chloride; potassium carbonate In N,N-dimethyl-formamide; toluene at 160℃; for 20h; Heck reaction; Inert atmosphere; | |
| With {2,6-bis[(di-1-piperidinylphosphino)amino]phenyl}palladium(II) chloride; potassium carbonate In N,N-dimethyl-formamide; toluene at 160℃; for 20h; Mizoroki-Heck reaction; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With potassium phosphate; bis(tricyclohexylphosphine)nickel(II) dichloride; water In 1,4-dioxane; toluene at 110℃; for 20h; Suzuki-Miyaura coupling; Inert atmosphere; | 89% |
-
-
58-72-0
Triphenylethylene

-
-
18084-97-4
chlorotriphenylethylene

| Conditions | Yield |
|---|---|
| With aluminium trichloride; benzeneseleninyl chloride In dichloromethane Ambient temperature; | 100% |
| With aluminium trichloride; benzeneseleninyl chloride In dichloromethane for 3h; Ambient temperature; | 100% |
| With phosphorus pentachloride at 190 - 200℃; |

| Conditions | Yield |
|---|---|
| With n-butyllithium; [(1,2-bis[(2,6-diisopropylphenyl)imino]acenaphthene)FeCl2]; hydrogen In toluene at 80℃; under 7500.75 Torr; for 16h; Sealed tube; | 99% |
| Stage #1: Triphenylethylene With lithium triethylborohydride; cobalt(II) bromide In tetrahydrofuran Inert atmosphere; Glovebox; Stage #2: With hydrogen In tetrahydrofuran at 20℃; under 1500.15 Torr; for 3h; Reagent/catalyst; | 99% |
| With iodine; hypophosphorous acid In acetic acid for 24h; Heating; | 97% |

| Conditions | Yield |
|---|---|
| With (NMe4)(Co-ortho-phenylenebis(N'-methyloxamidate)*2H2O*CH3CN; oxygen; pivalaldehyde In fluorobenzene for 2h; Ambient temperature; | 98% |
| With Oxone; edetate disodium; sodium hydrogencarbonate; 1,1-dioxotetrahydrothiopyran-4-one In acetonitrile for 6h; Ambient temperature; | 97% |
| With rhodium(II) acetate dimer; oxygen; isobutyraldehyde In acetone at 20℃; for 0.5h; | 95% |
-
-
58-72-0
Triphenylethylene

-
-
137742-63-3
2-fluoro-1,1,2-triphenylethanol

| Conditions | Yield |
|---|---|
| With water; N-fluorobis<(trifluoromethyl)sulfonyl>imide In dichloromethane at 0℃; for 1.5h; | 98% |
| With Selectfluor; water In acetonitrile for 0.0333333h; Microwave irradiation; regioselective reaction; | 95% |
| With water; 1-fluoro-4-hydroxy-1,4-diazoniabicyclo[2,2,2]octane-1,4-bis(tetrafluoroborate) In acetonitrile at 35℃; for 0.5h; | 98 % Spectr. |

| Conditions | Yield |
|---|---|
| With aluminum tri-bromide; benzeneseleninyl chloride In dichloromethane for 3h; Ambient temperature; | 97% |
| With bromine In chloroform at -78℃; for 6h; | 87% |
| Stage #1: Triphenylethylene With bromine In chloroform at 20℃; for 1.5h; Stage #2: With triethylamine In chloroform at 20℃; | 87% |
-
-
58-72-0
Triphenylethylene

-
-
125440-38-2
1,2-difluoro-1,1,2-triphenylethane

| Conditions | Yield |
|---|---|
| With (HF)nPy; N-fluorobis<(trifluoromethyl)sulfonyl>imide In dichloromethane at 0℃; for 2h; | 96% |
| With xenon difluoride; hydrogen fluoride In dichloromethane for 1h; | 44% |
| With silver fluoride; titanium(IV) oxide In acetonitrile for 48h; Ambient temperature; Irradiation; | 7 % Spectr. |
-
-
58-72-0
Triphenylethylene

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; tetrabutylammomium bromide; water-d2; cesium fluoride; silicon at 100℃; for 24h; | 96% |
| Stage #1: Triphenylethylene With sodium In tetrahydrofuran Inert atmosphere; Stage #2: With water-d2 In tetrahydrofuran Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With {Co(II)(DBF2)2(H2O)2} In acetonitrile at 20℃; for 18h; Sealed tube; UV-irradiation; Inert atmosphere; | 94% |
| With cyclohexane; iodine Irradiation.UV-Licht; | |
| palladium diacetate; palladium dichloride In 1,4-dioxane; acetic acid Heating; | |
| With iodine; oxygen In cyclohexane Irradiation; | |
| In ethanol Irradiation; |

| Conditions | Yield |
|---|---|
| With Selectfluor In acetonitrile for 0.025h; Microwave irradiation; regioselective reaction; | 94% |
| With Selectfluor In acetonitrile at 22℃; for 1h; Yield given; | |
| With 1-fluoro-4-hydroxy-1,4-diazoniabicyclo[2,2,2]octane-1,4-bis(tetrafluoroborate) In acetonitrile at 35℃; for 0.5h; | 97 % Spectr. |
| With 1-fluoro-4-hydroxy-1,4-diazoniabicyclo[2,2,2]octane-1,4-bis(tetrafluoroborate) In acetonitrile at 24℃; Kinetics; Fluorination; Nucleophilic addition; | |
| With Selectfluor |
Triphenylethylene Consensus Reports
Reported in EPA TSCA Inventory.
Triphenylethylene Specification
The Triphenylethylene with CAS registry number of 58-72-0 is also known as Benzilidenediphenylmethane. The IUPAC name is 1,2-Diphenylethenylbenzene. It belongs to product categories of Miscellaneous; Acyclic; Alkenes; Organic Building Blocks. Its EINECS registry number is 200-395-1. In addition, the formula is C20H16 and the molecular weight is 256.34. This chemical is a white to slightly beige powder and should be sealed in cool place.
Physical properties about Triphenylethylene are: (1)ACD/LogP: 7.00; (2)# of Rule of 5 Violations: 1; (3)ACD/LogD (pH 5.5): 7; (4)ACD/LogD (pH 7.4): 7; (5)ACD/BCF (pH 5.5): 122037.29; (6)ACD/BCF (pH 7.4): 122037.29; (7)ACD/KOC (pH 5.5): 152226.2; (8)ACD/KOC (pH 7.4): 152226.2; (9)#Freely Rotating Bonds: 3; (10)Index of Refraction: 1.645; (11)Molar Refractivity: 86.68 cm3; (12)Molar Volume: 239 cm3; (13)Surface Tension: 44.2 dyne/cm; (14)Density: 1.072 g/cm3; (15)Flash Point: 165 °C; (16)Enthalpy of Vaporization: 57.99 kJ/mol; (17)Boiling Point: 358.2 °C at 760 mmHg; (18)Vapour Pressure: 5.34E-05 mmHg at 25 °C.
Preparation of Triphenylethylene: it is prepared by reaction of benzophenone with benzylphosphonic acid diethyl ester. The reaction needs reagents NaH, 15-crown-5 and solvent tetrahydrofuran at the temperature of 0-25 °C for 3 hours. The yield is about 96%.
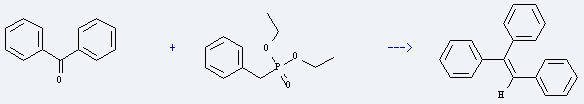
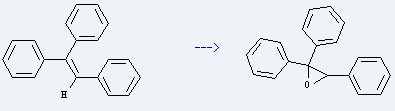
Uses of Triphenylethylene: it is used to produce triphenyl-oxirane. The reaction occurs with reagents oxone, tetrahydrothiopyran-1,1,4-trione, sodium bicarbonate, aq. Na2*EDTA and solvent acetonitrile at ambient temperature for 6 hours. The yield is about 97%.
When you are using this chemical, please be cautious about it. As a chemical, it is irritating to eyes and harmful if swallowed. What's more, it is very toxic to aquatic organisms and may cause long-term adverse effects in the aquatic environment. During using it, wear suitable protective clothing and gloves. If contact with eyes accidently, rinse immediately with plenty of water and seek medical advice. Besides, this material and its container must be disposed of as hazardous waste. Avoid release to the environment.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: C1=CC=C(C=C1)C=C(C2=CC=CC=C2)C3=CC=CC=C3
2. InChI: InChI=1S/C20H16/c1-4-10-17(11-5-1)16-20(18-12-6-2-7-13-18)19-14-8-3-9-15-19/h1-16H
3. InChIKey: MKYQPGPNVYRMHI-UHFFFAOYSA-N
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View