-
Name
beta-Bromostyrene
- EINECS 203-131-3
- CAS No. 103-64-0
- Article Data75
- CAS DataBase
- Density 1.44 g/cm3
- Solubility 56.624mg/L at 25℃
- Melting Point 7 °C(lit.)
- Formula C8H7Br
- Boiling Point 220.9 °C at 760 mmHg
- Molecular Weight 183.048
- Flash Point 101.7 °C
- Transport Information
- Appearance clear yellowish to brown liquid
- Safety 23-24/25-36/37
- Risk Codes 22
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Styrene, b-bromo- (6CI,7CI,8CI);(2-Bromoethenyl)benzene;(2-Bromovinyl)benzene;1-Bromo-2-phenylethene;1-Bromo-2-phenylethylene;2-Phenylethenyl bromide;Bromostyrolene;Bromstyrol;Hyacinth base;NSC 8047;Styryl bromide;a-Bromo-b-phenylethylene;b-Bromostyrene;w-Bromostyrene;
- PSA 0.00000
- LogP 3.05220
Synthetic route

| Conditions | Yield |
|---|---|
| With samarium In methanol at 45℃; for 6h; | 97% |
| With triethylamine; phosphonic acid diethyl ester for 4h; Ambient temperature; | 96% |
| With indium; ammonium chloride In ethanol; water for 16h; Heating; | 95% |

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; tetrabutylammomium bromide at 100℃; for 0.666667h; Hunsdiecker reaction; | 96% |

| Conditions | Yield |
|---|---|
| With acetic acid; zinc In dichloromethane for 0.3h; Heating; E/Z ratio 55:45; | 95% |

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene; tetraethylammonium bromide In dichloromethane at 20℃; for 4h; | 95% |
| With N-Bromosuccinimide; manganese (II) acetate tetrahydrate In water; acetonitrile at 20℃; | 92% |
| With N,N-dimethyl-formamide; potassium bromide; trichlorophosphate In acetonitrile at 20℃; Reagent/catalyst; Sonication; | 75% |
-
-
92933-32-9
(α,β-dibromohydrocinnamoyloxy)trimethylstannane

-
A
-
103-64-0
bromostyrene

| Conditions | Yield |
|---|---|
| at 180℃; | A 85% B n/a |

| Conditions | Yield |
|---|---|
| With potassium hydrogensulfate; hydroquinone In water at 150 - 185℃; | 84% |
-
-
75-25-2
Bromoform

-
-
100-52-7
benzaldehyde

-
A
-
103-64-0
bromostyrene

-
B
-
2612-41-1
α,α-(dibromomethyl)phenylmethanol

| Conditions | Yield |
|---|---|
| With 1,2-dimethoxyethane; titanium tetrachloride; magnesium In 1,2-dichloro-ethane at 0℃; for 3h; | A n/a B 80% |


| Conditions | Yield |
|---|---|
| Stage #1: Cinnamic acid With triethylamine In dichloromethane at 20℃; for 0.0833333h; Stage #2: N-Bromosuccinimide In dichloromethane for 1h; | 73% |
-
-
72591-21-0
2,2,2-tribromoethylbenzene

-
-
103-64-0
bromostyrene

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride; chromium(III) bromide; iron In tetrahydrofuran at 4℃; for 16h; | 64% |
| With sodium isopropanethiolate In methanol Heating; | 47% |

| Conditions | Yield |
|---|---|
| With sodium hypochlorite; bromine In water; acetonitrile at 20℃; for 8h; | A 47% B 18% |
-
-
83263-29-0
erythro-2,3-dibromo-3-phenylpropanol

-
A
-
103-64-0
bromostyrene

-
B
-
1504-53-6
3-bromo-3-phenyl-2-propenol

-
-
141811-76-9, 141811-77-0
(+/-)-threo-2-[bromo(phenyl)methyl]-oxirane

| Conditions | Yield |
|---|---|
| With sodium hydroxide In benzene for 5h; Product distribution; Heating; var. reaction conditions, with and without use of phase transfer reagents; | A 13% B 20% C 22% |


| Conditions | Yield |
|---|---|
| With sodium hydroxide lower-melting form; |
-
-
64-17-5
ethanol

-
-
6286-30-2, 31357-31-0, 54624-38-3, 91649-50-2, 99145-28-5, 113569-00-9, 113626-42-9
erythro-2,3-dibromo-3-phenylpropanoic acid

-
-
127-08-2
potassium acetate

-
-
103-64-0
bromostyrene


| Conditions | Yield |
|---|---|
| With water | |
| With aqueous alkali | |
| With ethanol; potassium acetate |

| Conditions | Yield |
|---|---|
| With zinc |
-
-
6286-30-2
2,3-dibromo-3-phenylpropanoic acid

-
-
121-69-7
N,N-dimethyl-aniline

-
-
71-43-2
benzene

-
A
-
292638-84-7
styrene

-
B
-
103-64-0
bromostyrene

-
C
-
621-82-9
Cinnamic acid

-
-
856380-20-6
2-bromo-1,4,11,12-tetrachloro-3-phenyl-1,2,3,4-tetrahydro-1,4-etheno-phenazine

-
A
-
103-64-0
bromostyrene

-
B
-
22213-14-5
1,2,3,4-tetrachloro-phenazine
-
-
3712-44-5
2,2,2-tribromo-1,3,2-benzodioxaphosphole

-
-
122-78-1
phenylacetaldehyde

-
A
-
2612-38-6
1,1-dibromo-2-phenylethane

-
B
-
103-64-0
bromostyrene

| Conditions | Yield |
|---|---|
| In dichloromethane for 2h; Ambient temperature; Yield given. Yields of byproduct given; |

-
-
39598-55-5
(bromomethylene)triphenylphosphorane

-
-
100-52-7
benzaldehyde

-
A
-
103-64-0
bromostyrene

-
B
-
536-74-3
phenylacetylene

| Conditions | Yield |
|---|---|
| THF; Yield given. Multistep reaction; |

-
-
120809-73-6
1,1-dibromo-2-phenyl-2-(trimethylsiloxy)ethane

-
A
-
103-64-0
bromostyrene

-
B
-
35952-70-6
(2-Bromo-1-phenyl-ethoxy)-trimethyl-silane

-
C
-
57044-58-3
(E)/(Z)-β-Trimethylsiloxystyrene

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sec.-butyllithium 1.) THF, ether, pentane, -130 deg C, 4 h; 2.) CH3OH; Yield given. Multistep reaction. Yields of byproduct given; |

-
-
120809-73-6
1,1-dibromo-2-phenyl-2-(trimethylsiloxy)ethane

-
A
-
103-64-0
bromostyrene

-
B
-
57044-58-3
(E)/(Z)-β-Trimethylsiloxystyrene

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sec.-butyllithium 1.) THF, ether, pentane, -115 deg C, 4 h; 2.) CH3OH; Yield given. Multistep reaction. Yields of byproduct given; |
-
-
292638-84-7
styrene

-
-
67-68-5
dimethyl sulfoxide

-
A
-
1075-20-3
(+/-)-4-phenyl-[1,3]-dioxolane

-
B
-
1074-12-0
phenylglyoxal hydrate

-
C
-
103-64-0
bromostyrene

-
D
-
2425-28-7
2-Bromo-1-phenylethanol

-
E
-
98-86-2
acetophenone

-
F
-
102921-26-6, 93-52-7
(1,2-dibromoethyl)benzene

| Conditions | Yield |
|---|---|
| With bromine Product distribution; Mechanism; 1) 15 deg C, 20 min; 2) rt, 1 h; 3) 80 deg C, 21 h; further reagent: HBr; | A n/a B 32 % Spectr. C n/a D n/a E n/a F n/a G n/a |


| Conditions | Yield |
|---|---|
| Einwirkung auf zimtsaures Alkali; |


-
-
103-64-0
bromostyrene

| Conditions | Yield |
|---|---|
| With sodium carbonate |

-
-
103-64-0
bromostyrene

| Conditions | Yield |
|---|---|
| With water; bromine |

-
-
103-64-0
bromostyrene

| Conditions | Yield |
|---|---|
| With sunlight lower-melting form; |

-
-
103-64-0
bromostyrene

| Conditions | Yield |
|---|---|
| With sunlight higher-melting form; |

-
A
-
292638-84-7
styrene

-
B
-
103-64-0
bromostyrene

| Conditions | Yield |
|---|---|
| With ethanol; zinc |
-
-
140-10-3
(E)-3-phenylacrylic acid

-
-
7732-18-5
water

-
A
-
103-64-0
bromostyrene

-
B
-
34882-18-3
2-bromo-3-hydroxy-3-phenyl-propanoic acid

-
C
-
6622-79-3
2-bromo-3-chloro-3-phenyl-propionic acid

| Conditions | Yield |
|---|---|
| at 48℃; |


| Conditions | Yield |
|---|---|
| With potassium tert-butylate | 100% |
| With sodium hexamethyldisilazane In 1,2-dimethoxyethane at 20℃; for 1h; | 92% |
| With potassium tert-butylate In 1-methyl-pyrrolidin-2-one at 50℃; for 0.166667h; Schlenk technique; Inert atmosphere; | 86% |

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; water In acetonitrile at 20℃; | 100% |
-
-
103-64-0
bromostyrene

-
-
1294447-76-9
1,3-bis(2,6-difluorophenyl)triazene

| Conditions | Yield |
|---|---|
| With potassium hexafluorophosphate; tert-butylhypochlorite In dichloromethane at -78 - 20℃; Darkness; | 100% |
-
-
103-64-0
bromostyrene

-
-
50487-71-3
4-methyl-N-(2-propenyl)benzenesulfonamide

-
-
1428946-22-8
(E)-N-allyl-N-styryl-4-methylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With copper(l) iodide; caesium carbonate; N,N`-dimethylethylenediamine In tetrahydrofuran Schlenk technique; Inert atmosphere; Reflux; | 100% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; tetrabutylammonium acetate; potassium carbonate; triphenylphosphine In water; dimethyl sulfoxide at 100℃; for 24h; Catalytic behavior; Reagent/catalyst; Temperature; Time; Sonogashira Cross-Coupling; Inert atmosphere; Green chemistry; | 99% |
| With iodo(4,5-bis(diphenylphosphano)-9,9-dimethylxanthene)copper(I); caesium carbonate In 1,4-dioxane at 135℃; for 24h; Inert atmosphere; | 98% |
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; diethylamine at 25℃; for 12h; | 82% |
-
-
103-64-0
bromostyrene

-
-
1446446-71-4
(E)-3-((E)-1,3-diphenylallylidene)-5,5-dimethyl-1,2-oxaborolan-2-ol

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); sodium hydroxide In 1,4-dioxane; water at 50℃; for 3h; Suzuki-Miyaura Coupling; | 99% |

| Conditions | Yield |
|---|---|
| With N,N′-Bis(2-pyridylmethylidene)-1,2-trans-(R,R + S,S)-cyclohexanediamine; hydrogen; iron(II) chloride In tetrahydrofuran at -20 - 20℃; under 37503.8 Torr; for 18h; | 99% |

| Conditions | Yield |
|---|---|
| With lithium hexamethyldisilazane In 1,2-dimethoxyethane at 20℃; for 3h; regioselective reaction; | 99% |
-
-
103-64-0
bromostyrene

-
-
78999-89-0
N-(diphenylmethylene)-1-(4-methoxyphenyl)methanamine

| Conditions | Yield |
|---|---|
| With lithium hexamethyldisilazane In 1,2-dimethoxyethane at 20℃; for 3h; regioselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With lithium hexamethyldisilazane In 1,2-dimethoxyethane at 20℃; for 6h; regioselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With lithium hexamethyldisilazane In 1,2-dimethoxyethane at 20℃; for 1.5h; regioselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With lithium hexamethyldisilazane In 1,2-dimethoxyethane at 20℃; for 1.5h; regioselective reaction; | 99% |
-
-
103-64-0
bromostyrene

-
-
70978-37-9
1-(azidomethyl)-4-methoxybenzene

-
-
126800-00-8
1-(4-methoxybenzyl)-4-phenyl-1H-1,2,3-triazole

| Conditions | Yield |
|---|---|
| With piperidine; copper(II) oxide at 110℃; for 12h; | 99% |
-
-
103-64-0
bromostyrene

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); sodium hydroxide In 1,4-dioxane; water at 100℃; for 16h; Inert atmosphere; Schlenk technique; | 99% |

| Conditions | Yield |
|---|---|
| With N,N-diethylaniline; tetrakis(triethylphosphite)nickel(0) In N,N-dimethyl-formamide at 70℃; for 23h; | 98% |
| at 140 - 160℃; Yield given; | |
| With sodium hydroxide; trans-bromo(phenyl)bis(triphenylphosphine)palladium(II); cetyltributylphosphonium bromide; triphenylphosphine In toluene at 80℃; for 9h; | 80 % Chromat. |

| Conditions | Yield |
|---|---|
| With sodium bromite; tributyltin chloride In dichloromethane; water Ambient temperature; | 98% |
-
-
20154-03-4
3-(trifluoromethyl)pyrazole

-
-
103-64-0
bromostyrene

| Conditions | Yield |
|---|---|
| With copper(l) iodide; caesium carbonate In acetonitrile at 50℃; for 30h; | 98% |

| Conditions | Yield |
|---|---|
| With iodo(4,5-bis(diphenylphosphano)-9,9-dimethylxanthene)copper(I); caesium carbonate In 1,4-dioxane at 135℃; for 24h; Inert atmosphere; | 98% |
| With CuI(xantphos); caesium carbonate In N,N-dimethyl-formamide at 135℃; for 1h; Sonogashira type reaction; Inert atmosphere; Microwave irradiation; | 36% |
-
-
103-64-0
bromostyrene

-
-
768-60-5
4-methoxyphenylacetylen

-
-
116156-19-5
(E)-1-methoxy-4-(4-phenylbutyl-3-en-1-yn-1-yl)benzene

| Conditions | Yield |
|---|---|
| With copper(l) iodide; tetrabutylammonium acetate; potassium carbonate; triphenylphosphine In water; dimethyl sulfoxide at 100℃; for 24h; Sonogashira Cross-Coupling; Inert atmosphere; | 98% |

-
-
103-64-0
bromostyrene

-
-
40595-34-4
(E)-trimethyl(3-phenylallyl)silane

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride In toluene at 20℃; for 1h; Schlenk technique; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With lithium hexamethyldisilazane In 1,2-dimethoxyethane at 20℃; for 0.5h; Reagent/catalyst; Solvent; Time; Concentration; regioselective reaction; | 98% |
-
-
103-64-0
bromostyrene

-
-
824-79-3
sodium 4-methylbenzenesulfinate

-
-
16212-08-1
1-methyl-4-[(E)-2-phenylethenesulfonyl]benzene

| Conditions | Yield |
|---|---|
| With (1,2-dimethoxyethane)dichloronickel(II); C48H56IrN4(1+)*F6P(1-); 4,4'-di-tert-butyl-2,2'-bipyridine In N,N-dimethyl-formamide at 20℃; for 24h; Inert atmosphere; Irradiation; Sealed tube; | 98% |

| Conditions | Yield |
|---|---|
| With piperidine; copper(II) oxide at 110℃; for 12h; | 98% |

| Conditions | Yield |
|---|---|
| With gallium(III) trichloride; 2,4-lutidine In 2-methyltetrahydrofuran; isopropyl alcohol at 85℃; for 4h; Reagent/catalyst; Solvent; Inert atmosphere; | 97.6% |


| Conditions | Yield |
|---|---|
| With triisopropanolamine; copper acetylacetonate; palladium(II) trifluoroacetate; 1-(2-ethoxyethyl)-3-methylimidazolium trifluoromethanesulfonimidide In N,N-dimethyl-formamide at 60℃; for 10h; Reagent/catalyst; Solvent; | 97.5% |

| Conditions | Yield |
|---|---|
| With triisopropanolamine; copper acetylacetonate; palladium(II) trifluoroacetate; 1-(2-ethoxyethyl)-3-methylimidazolium trifluoromethanesulfonimidide In N,N-dimethyl-formamide at 80℃; for 6h; Reagent/catalyst; Solvent; | 97.4% |

| Conditions | Yield |
|---|---|
| With triisopropanolamine; copper acetylacetonate; palladium(II) trifluoroacetate; 1-(2-ethoxyethyl)-3-methylimidazolium trifluoromethanesulfonimidide In N,N-dimethyl-formamide at 70℃; for 8h; Reagent/catalyst; Solvent; | 97.3% |

| Conditions | Yield |
|---|---|
| Stage #1: oct-1-ene With 9-borabicyclo[3.3.1]nonane dimer In tetrahydrofuran at 20℃; for 17h; Stage #2: bromostyrene With potassium phosphate; 2H(1+)*Cl4Pd(2-)*2H3N; poly[N-isopropylacrylamide-co-diphenyl(4'-styryl)phosphine] In tetrahydrofuran; 1,4-dioxane at 100℃; for 1.5h; Suzuki-Miyaura reaction; | 97% |

| Conditions | Yield |
|---|---|
| Stage #1: bromostyrene With tert.-butyl lithium In diethyl ether; pentane at 25℃; for 0.5h; Stage #2: With indium(III) chloride In diethyl ether; pentane at 25℃; for 0.5h; Stage #3: ethyl iodoacetae With triethyl borane In diethyl ether; hexane; pentane at 25℃; for 14h; | 97% |
| Stage #1: bromostyrene With tert.-butyl lithium In diethyl ether; pentane at -78℃; for 1h; Stage #2: With indium(III) chloride In tetrahydrofuran; diethyl ether; pentane at -78℃; for 1h; Stage #3: ethyl iodoacetae In tetrahydrofuran; diethyl ether; pentane at 20℃; Irradiation; Further stages.; | 83% |
beta-Bromostyrene Consensus Reports
Reported in EPA TSCA Inventory.
beta-Bromostyrene Specification
The β-Bromostyrene is an organic compound with the formula C8H7Br. The IUPAC name of this chemical is 2-bromoethenylbenzene. With the CAS registry number 103-64-0, it is also named as 1-Bromo-2-phenylethene. The product's category is Pharmaceutical Intermediates. Besides, it is clear yellowish to brown liquid, which should be stored in a cool and ventilated place. It is used as a fragrance intermediates.
Physical properties about β-Bromostyrene are: (1)ACD/LogP: 2.99; (2)ACD/LogD (pH 5.5): 2.99; (3)ACD/LogD (pH 7.4): 2.99; (4)ACD/BCF (pH 5.5): 110.05; (5)ACD/BCF (pH 7.4): 110.05; (6)ACD/KOC (pH 5.5): 1006.87; (7)ACD/KOC (pH 7.4): 1006.87; (8)#Freely Rotating Bonds: 1; (9)Index of Refraction: 1.63; (10)Molar Refractivity: 45.24 cm3; (11)Molar Volume: 127 cm3; (12)Polarizability: 17.93×10-24cm3; (13)Surface Tension: 41.5 dyne/cm; (14)Density: 1.44 g/cm3; (15)Flash Point: 101.7 °C; (16)Enthalpy of Vaporization: 43.87 kJ/mol; (17)Boiling Point: 220.9 °C at 760 mmHg; (18)Vapour Pressure: 0.164 mmHg at 25°C.
Preparation: this chemical can be prepared by β,β-dibromo-styrene. This reaction will need reagent HP(O)(OEt)2, Et3N. The reaction time is 4 hours at ambient temperature. The yield is about 96%.

Uses of β-Bromostyrene: it can be used to produce styrylsulfanyl-benzene at temperature of 135 °C. It will need reagent 1-methyl-2-pyrrolidone with reaction time of 2 hours. The yield is about 95%.
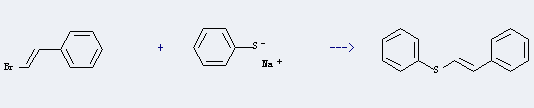
When you are using this chemical, please be cautious about it as the following:
It is harmful if swallowed. When you are using it, wear suitable protective clothing and gloves, do not breathe gas/fumes/vapour/spray (appropriate wording to be specified by the manufacturer) and avoid contact with skin and eyes.
You can still convert the following datas into molecular structure:
(1)SMILES: BrC=Cc1ccccc1
(2)InChI: InChI=1/C8H7Br/c9-7-6-8-4-2-1-3-5-8/h1-7H
(3)InChIKey: YMOONIIMQBGTDU-UHFFFAOYAF
(4)Std. InChI: InChI=1S/C8H7Br/c9-7-6-8-4-2-1-3-5-8/h1-7H
(5)Std. InChIKey: YMOONIIMQBGTDU-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | oral | 1250mg/kg (1250mg/kg) | Food and Cosmetics Toxicology. Vol. 11, Pg. 1043, 1973. |
Related Products
- beta-Bromostyrene
- 1036401-98-5
- 1036401-99-6
- 103646-25-9
- 103646-29-3
- 103646-82-8
- 1036468-34-4
- 10364-68-8
- 10364-69-9
- 10364-94-0
- 103-65-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View