-
Name
CIS-2-BUTENE
- EINECS 209-673-7
- CAS No. 590-18-1
- Article Data767
- CAS DataBase
- Density 0.636 g/cm3
- Solubility Insoluble in water
- Melting Point -139 °C(lit.)
- Formula C4H8
- Boiling Point 3.719 °C at 760 mmHg
- Molecular Weight 56.1075
- Flash Point -99 °F
- Transport Information UN 1012 2.1
- Appearance colorless liquefied petroleum gas.
- Safety 9-16-33
- Risk Codes 12
-
Molecular Structure
-
Hazard Symbols
 F+
F+
- Synonyms 2-Butene, (Z)-;High-boiling butene-2;cis-1,2-Dimethylethylene;beta-cis-Butylene;beta-cis-Butylene;
- PSA 0.00000
- LogP 1.58240
Synthetic route

| Conditions | Yield |
|---|---|
| In neat (no solvent) heating at 50°C for a few hours; same result in CCl4 at 50°C; | A 100% B n/a C n/a D n/a |


| Conditions | Yield |
|---|---|
| In not given 50°C; | A 98% B n/a |


-
A
-
106-98-9
1-butylene

-
B
-
590-18-1
(Z)-2-Butene

-
C
-
624-64-6
trans-2-Butene

-
D
-
74-85-1
ethene

-
E
-
106-97-8
n-butane

| Conditions | Yield |
|---|---|
| In toluene thermal decompn. at 95°C (70 h); | A 0.4% B 0.6% C 1.1% D 97.8% E 0.1% |
| In toluene thermal decompn. at 95°C (23 h); | A 8% B 20.9% C 46.3% D 20.2% E 4.6% |


| Conditions | Yield |
|---|---|
| Ni(DMPMNBu)Cl2 In toluene at 30 - 60℃; under 15201 Torr; Product distribution / selectivity; Autoclave; Gas phase; | A 93.7% B 5.1% |
| yttrium; nickel(II) at 199.9℃; Product distribution; various Ni-substituted catalysts; | A 7.1% B 90% |
| With C16H19Br2N4NiP In toluene at 30℃; under 6000.6 Torr; for 0.5h; Catalytic behavior; Time; Reagent/catalyst; Temperature; |

-
A
-
106-98-9
1-butylene

-
B
-
590-18-1
(Z)-2-Butene

-
C
-
624-64-6
trans-2-Butene

-
D
-
74-85-1
ethene

-
E
-
106-99-0
buta-1,3-diene

| Conditions | Yield |
|---|---|
| In toluene thermal decompn. at 95°C (23 h); | A 93.1% B 1.3% C 0.3% D 0.5% E 4.8% |


-
A
-
106-98-9
1-butylene

-
B
-
590-18-1
(Z)-2-Butene

-
-
16971-06-5, 19696-06-1
trans-hydridoiodobis(triethylphosphine)platinum(II)

-
D
-
624-64-6
trans-2-Butene

| Conditions | Yield |
|---|---|
| In acetone Kinetics; at 283.66-313.16 K; NMR; | A 91.8% B 5.9% C n/a D 3.1% |


-
A
-
106-98-9
1-butylene

-
B
-
590-18-1
(Z)-2-Butene

-
-
37809-11-3
trans-bis(triethylphosphine)(hydrido)(selenocyanato) platinum(II)

-
D
-
624-64-6
trans-2-Butene

| Conditions | Yield |
|---|---|
| In acetone Kinetics; at 298.16 K; NMR; | A 90.6% B 5.4% C n/a D 4% |


| Conditions | Yield |
|---|---|
| In benzene-d6 Kinetics; at 167 +/- 1°C; | A 90% B <5 |

| Conditions | Yield |
|---|---|
| In benzene-d6 Kinetics; thermolysis at 167+/-1°C; | A 6% B 90% |

| Conditions | Yield |
|---|---|
| In benzene at 50℃; for 2h; | A n/a B 89% |

-
A
-
106-98-9
1-butylene

-
B
-
590-18-1
(Z)-2-Butene

-
C
-
624-64-6
trans-2-Butene

-
D
-
106-99-0
buta-1,3-diene

-
E
-
106-97-8
n-butane

| Conditions | Yield |
|---|---|
| In toluene thermal decompn. at 95°C (70 h); | A 88.9% B 3.3% C 2.8% D 4.2% E 0.8% |


| Conditions | Yield |
|---|---|
| In toluene heating at 80°C; recrystd. from benzene; elem. anal.; | A 88% B n/a |


-
A
-
106-98-9
1-butylene

-
B
-
590-18-1
(Z)-2-Butene

-
C
-
624-64-6
trans-2-Butene

-
D
-
74-98-6
propane

-
E
-
74-85-1
ethene

| Conditions | Yield |
|---|---|
| In toluene thermal decompn. at 60°C (15 h); further product: cyclobutane; | A 85.9% B 3.4% C 3.6% D 1% E 5.7% |
| In toluene thermal decompn. at 95°C (15 h); further product: cyclobutane; | A 58.7% B 2.5% C 2.5% D 1% E 36.3% |


| Conditions | Yield |
|---|---|
| In benzine at 20 - 50℃; for 2.16667h; | A n/a B 85% |


-
-
590-18-1
(Z)-2-Butene

| Conditions | Yield |
|---|---|
| With lead(IV) acetate at 0℃; for 0.5h; | 83% |
-
-
63950-40-3, 25395-92-0
bis(diethylcarbamodithioato-S,S')oxomolybdenum

-
-
925669-95-0
2,3-cis-epoxybutane

-
A
-
590-18-1
(Z)-2-Butene

| Conditions | Yield |
|---|---|
| In toluene N2 atmosphere, 130°C, 45 h; olefine: GC; pptd. complex: elem. anal.; | A 83% B n/a |


-
A
-
106-98-9
1-butylene

-
B
-
590-18-1
(Z)-2-Butene

-
-
12086-27-0
trans-bis(triethylphosphine)(H)(thiocyanato) platinum(II)

-
E
-
624-64-6
trans-2-Butene

| Conditions | Yield |
|---|---|
| In acetone Kinetics; at 298.16 K; | A 81% B 15.8% C n/a D n/a E 4% |


-
A
-
106-98-9
1-butylene

-
B
-
590-18-1
(Z)-2-Butene

-
-
18660-33-8, 20436-51-5
trans-bis(triethylphosphine)(hydrido)(Br) platinum(II)

-
D
-
624-64-6
trans-2-Butene

| Conditions | Yield |
|---|---|
| In acetone Kinetics; at 298.16 K; | A 80% B 16.5% C n/a D 3.5% |

-
-
3017-71-8
(E)-2-bromobut-2-ene

-
-
126430-46-4
benzyl 4-bromobutanoate

-
A
-
18265-39-9
(E,E)-3,4-dimethylhexa-2,4-diene

-
B
-
590-18-1
(Z)-2-Butene

-
C
-
42413-23-0
octanedioic acid dibenzyl ester

-
D
-
103-37-7
benzyl butanoate

-
E
-
1365610-69-0
(E)-benzyl 5-methylhept-5-enoate

| Conditions | Yield |
|---|---|
| With pyridine; 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone; 4,4'-Dimethoxy-2,2'-bipyridin; NiI2*3.5H2O; sodium iodide; zinc at 20 - 60℃; for 22h; chemoselective reaction; | A n/a B n/a C n/a D n/a E 79% |

-
-
73448-09-6
octacarbonyl(μ-methylene)diiron

-
-
187737-37-7
propene

-
A
-
106-98-9
1-butylene

-
B
-
590-18-1
(Z)-2-Butene

-
C
-
12192-99-3
Fe(CO)4(η2-CH2CHCH3)

-
D
-
624-64-6
trans-2-Butene

-
E
-
115-11-7
isobutene

| Conditions | Yield |
|---|---|
| In benzene High Pressure; autoclave charged with complex, C6H6 and propylene (50 psi), heated at 55°C for 2 h; cooled to 0°C, purged with N2, not isolated, detected by IR; | A 1% B 5% C n/a D 15% E 78% |


| Conditions | Yield |
|---|---|
| Pd-Ca-X zeolite at 49.9℃; Product distribution; var. temp.; determination of cat. activity; | A 5% B 72% C 25% |
| Ni(C5H7S2)PBu3Cl at -20℃; Product distribution; further Ni-catalysts; various temperatures; further oligomeric products; | A 4% B 27% C 69% |
| With tetrabutylammonium perchlorate; bis(triphenylphosphine)nickel(II) chloride In various solvent(s) at 25℃; under 3750.3 Torr; for 24h; Product distribution; electrochemical reduction process, effect of anode, supporting electrolyte, ligand, temperature and pressure; | A 7% B 28% C 65% |

-
-
106-99-0
buta-1,3-diene

-
A
-
106-98-9
1-butylene

-
B
-
590-18-1
(Z)-2-Butene

-
C
-
624-64-6
trans-2-Butene

-
D
-
106-97-8
n-butane

| Conditions | Yield |
|---|---|
| With hydrogen; palladium In various solvent(s) at 40℃; under 3040 Torr; Kinetics; Product distribution; Further Variations:; Solvents; solvent-free; | A 72% B n/a C n/a D n/a |
| With hydrogen; palladium dichloride In N,N-dimethyl-formamide under 18751.5 Torr; for 0.383333h; Product distribution; Ambient temperature; various time; | A 50% B 2.5% C 23% D 1% |
| With hydrogen; LaPd3 at -38.1 - -0.1℃; Product distribution; Thermodynamic data; other catalyst; Ea; |


| Conditions | Yield |
|---|---|
| In toluene decompn. of the Pd compound induced by n-Bu2O*BF3; reactants (ratio n-Bu2O*BF3:Pd = 2:1) mixed at -78°C and then the reaction mixt. warmed to room temp.; | A 4.7% B 20.9% C 70.2% D 4.2% |

-
-
89308-86-1
cis-{Pt(PEt3)2(n-butyl)Cl}

-
A
-
106-98-9
1-butylene

-
B
-
590-18-1
(Z)-2-Butene

-
-
16842-17-4, 20436-52-6, 89254-73-9
trans-chlorohydridobis(triethylphosphine)platinum(II)

-
D
-
624-64-6
trans-2-Butene

| Conditions | Yield |
|---|---|
| In acetone Kinetics; at 298.16 K; NMR; | A 70.2% B 25.8% C n/a D 4% |

-
-
16054-34-5
(Z)-1,4-bis(trimethylsilyl)-2-butene

-
A
-
106-98-9
1-butylene

-
B
-
590-18-1
(Z)-2-Butene

-
C
-
60171-48-4
trans-1-Butenyltrimethylsilan

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In N,N,N,N,N,N-hexamethylphosphoric triamide at 60℃; for 3h; | A 10% B 70% C 20% |
| With potassium tert-butylate In N,N,N,N,N,N-hexamethylphosphoric triamide at 60℃; for 3h; | A 10% B 70% C 10% |


| Conditions | Yield |
|---|---|
| In acetone Kinetics; at 298.16 K; | A 69.4% B 26.2% C n/a D 4.4% |


-
A
-
106-98-9
1-butylene

-
B
-
590-18-1
(Z)-2-Butene

-
-
17501-31-4
trans-bis(triethylphosphine)(hydrido)(nitrito) platinum(II)

-
D
-
624-64-6
trans-2-Butene

| Conditions | Yield |
|---|---|
| In acetone Kinetics; at 298.16 K; NMR; | A 69.1% B 27.6% C n/a D 3.3% |


| Conditions | Yield |
|---|---|
| With tetrakis(acetonitrile)palladium(II) tetrafluoroborate In acetonitrile for 1h; Ambient temperature; | A 28% B 67% |
| at 26.9 - 926.9℃; thermodynamisch berechnete Gleichgewichte; | |
| at 24.9 - 1226.9℃; thermodynamisch berechnete Gleichgewichte; |
-
-
71-55-6
1,1,1-trichloroethane

-
A
-
590-18-1
(Z)-2-Butene

-
B
-
75-34-3
1,1-dichloroethane

-
C
-
74-84-0
ethane

-
D
-
74-85-1
ethene

| Conditions | Yield |
|---|---|
| With Tris buffer; iron; sodium chloride pH=7.5; Kinetics; Product distribution; Further Variations:; Reagents; Dehalogenation; dimerization; | A n/a B 67% C n/a D n/a |

-
-
17486-13-4
(Z)-crotyltrimethylsilane

-
A
-
106-98-9
1-butylene

-
B
-
590-18-1
(Z)-2-Butene

-
C
-
60171-48-4
trans-1-Butenyltrimethylsilan

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In N,N,N,N,N,N-hexamethylphosphoric triamide at 60℃; for 3h; | A 10% B 65% C 25% |

-
-
67-56-1
methanol

-
-
590-18-1
(Z)-2-Butene

-
-
91491-61-1
pyridine-2-selenenyl bromide

-
-
96818-37-0, 96818-38-1
2-((1R,2R)-2-Methoxy-1-methyl-propylselanyl)-pyridine

| Conditions | Yield |
|---|---|
| at -50 - 20℃; for 4h; | 100% |


| Conditions | Yield |
|---|---|
| With tin(IV) chloride In dichloromethane at -78℃; var. reag.: var. erythro/ threo ratio; | 100% |
-
-
590-18-1
(Z)-2-Butene

-
-
924-53-8
isopropyl glyoxalate

-
-
344750-29-4
2-Hydroxy-3-methyl-pent-4-enoic acid isopropyl ester

| Conditions | Yield |
|---|---|
| With tin(IV) chloride In dichloromethane at -78℃; var. reag; var. erythro/ threo ratio; | 100% |
-
-
590-18-1
(Z)-2-Butene

-
-
250665-62-4
1,1,4,4-tetrakis(trimethylsilyl)butane-1,4-diylsilylene

| Conditions | Yield |
|---|---|
| In hexane at 10℃; for 3h; Irradiation; | 100% |

| Conditions | Yield |
|---|---|
| In hexane at 30℃; for 6h; Inert atmosphere; Darkness; | 100% |
-
-
590-18-1
(Z)-2-Butene

| Conditions | Yield |
|---|---|
| Stage #1: (Z)-2-Butene With n-butyllithium; potassium tert-butylate In tetrahydrofuran; hexane at -50℃; for 0.5h; Stage #2: With triisopropylborane In tetrahydrofuran; hexane at -78℃; for 2h; Stage #3: (1R,2S,3R,4S)-2-phenyl-1,7,7-trimethylbornanediol In tetrahydrofuran; hexane at 20℃; for 0.5h; | 99% |
-
-
590-18-1
(Z)-2-Butene

-
-
2923-28-6
silver trifluoromethanesulfonate

-
-
157533-99-8, 157533-74-9
cyclopentadienyl(1,4-diisopropyl-1,3-diazabutadiene)(η2-cis-2-butene)ruthenium trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| In tetrahydrofuran under N2; equimolar amts. Ru-complex and Ag salt dissolved in THF, mixt. stirred (room temp., 15 min), formation of intermediate, suspn. filtered, red-brown soln. cooled to 0°C, stream of butene slowly passedthrough the soln.; isolated; | 99% |


| Conditions | Yield |
|---|---|
| With sodium azide In dimethyl sulfoxide Ambient temperature; | 98% |
-
-
590-18-1
(Z)-2-Butene

-
-
81207-06-9
1-methyl-1,2-dithiolanium fluoroborate

-
-
81207-23-0
trans-1,2,3-trimethyl-1,4-dithiepanium BF4(1-)

| Conditions | Yield |
|---|---|
| In nitromethane at 0℃; | 98% |
-
-
590-18-1
(Z)-2-Butene

-
-
81207-07-0
1-methyl-1,2-dithianium fluoroborate

-
-
81207-27-4
trans-1,2,3-trimethyl-1,4-dithiocanium BF4(1-)

| Conditions | Yield |
|---|---|
| In nitromethane at 0℃; | 98% |
-
-
590-18-1
(Z)-2-Butene

-
-
882-33-7
diphenyldisulfane

-
-
63298-00-0, 132685-32-6, 132917-25-0
(+/-)-threo-2,3-bis(phenylthio)butane

| Conditions | Yield |
|---|---|
| With boron trifluoride dimethyl etherate In nitromethane; dichloromethane at 0℃; for 0.5h; | 98% |
-
-
590-18-1
(Z)-2-Butene

-
-
400737-27-1
(R)-4-Methoxy-3-(4-methoxy-benzyloxy)-butyraldehyde

-
-
400737-30-6
(3R,4R,6R)-7-Methoxy-6-(4-methoxy-benzyloxy)-3-methyl-hept-1-en-4-ol

| Conditions | Yield |
|---|---|
| Multistep reaction; | 98% |

| Conditions | Yield |
|---|---|
| In diethyl ether at 0℃; | 97% |

| Conditions | Yield |
|---|---|
| With (acetylacetonato)dicarbonylrhodium (l); 1,2,4,5-tetraisopropylbenzene; C43H53O8P; hydrogen In toluene under 37503.8 Torr; for 12h; Catalytic behavior; Pressure; regioselective reaction; | 96.5% |
| With acetylacetonatodicarbonylrhodium(l); 3,3'-di-tert-butyl-5,5'-dimethoxy-[1,1'-biphenyl]-2,2'-diyltetrakis(2,4-dimethylphenyl)bis(phosphite); bis-(2,2,6,6-tetramethyl-4-piperidinyl) sebacate; hydrogen In toluene at 120℃; under 15001.5 Torr; for 12h; Catalytic behavior; Reagent/catalyst; Autoclave; | 95% |
| With dicarbonylacetylacetonato rhodium (I); trans-1,12-bis((di(1H-pyrrol-1-yl)phosphino)oxy)-5,5a,6,7,7a,8-hexahydrocyclopenta[1,2-b:1,5-b']dichromene; hydrogen In toluene at 110℃; under 3750.38 - 7500.75 Torr; for 15h; Glovebox; Autoclave; regioselective reaction; |
-
-
590-18-1
(Z)-2-Butene

-
-
280-64-8
9-bora-bicyclo[3.3.1]nonane

-
-
53317-06-9
B-sec-butyl-9-borabicyclo<3.3.1>nonane

| Conditions | Yield |
|---|---|
| In pentane at 0 - 20℃; | 96% |
| In tetrahydrofuran for 2h; Heating; |
-
-
590-18-1
(Z)-2-Butene

-
-
2714-60-5
tetrakis(trifluoromethyl)diphosphine

-
-
34250-87-8
2,3-bis(di(trifluoromethyl)phosphino)butane

| Conditions | Yield |
|---|---|
| 55°C (120 h); | 95% |
| Irradiation (UV/VIS); 20°C (120 h); | 89% |
| Irradiation; |

| Conditions | Yield |
|---|---|
| (2,6-Ph2C6H3O)2W(Cl)=CHC(CH3)3*OEt2 In chlorobenzene at 20℃; for 15h; | 95% |

| Conditions | Yield |
|---|---|
| In chloroform at 80℃; for 1h; Product distribution; stereochemistry; other olefins; | 95% |
| In chloroform at 80℃; for 1h; | 95% |
-
-
590-18-1
(Z)-2-Butene

-
-
5799-67-7
dimethyl(methylthio)sulfonium tetrafluoroborate

-
-
81206-99-7
C7H17S2(1+)*BF4(1-)

| Conditions | Yield |
|---|---|
| In nitromethane at 0℃; | 95% |
-
-
590-18-1
(Z)-2-Butene

-
-
51558-78-2
phenylselenenyl trifluoroacetate

-
-
51558-82-8, 51558-83-9
Trifluoro-acetic acid (1R,2R)-1-methyl-2-phenylselanyl-propyl ester

| Conditions | Yield |
|---|---|
| In dichloromethane for 0.25h; | 95% |
-
-
848617-93-6
(+/-)-B-methoxy-10-trimethylsilyl-9-borabicyclo[3.3.2]decane

-
-
590-18-1
(Z)-2-Butene

| Conditions | Yield |
|---|---|
| With n-butyllithium; trimethylsilyl trifluoromethanesulfonate; potassium tert-butylate In tetrahydrofuran; diethyl ether t-BuOK/THF; n-BuLi; borabicyclodecane, Et2O, -78°C, 15 min; TMSOTf, -78°C, 15 min; | 95% |

-
-
590-18-1
(Z)-2-Butene

| Conditions | Yield |
|---|---|
| In dichloromethane room temp., 12 h; elem. anal.; | 95% |

-
-
590-18-1
(Z)-2-Butene

-
-
1106964-40-2
1-methylprop-2-ene-1-sulfonyl chloride

| Conditions | Yield |
|---|---|
| Stage #1: (Z)-2-Butene With sulfur dioxide; boron trichloride In dichloromethane at -196 - -20℃; for 3h; Stage #2: With N-chloro-succinimide In dichloromethane at -20℃; for 2h; | 95% |

| Conditions | Yield |
|---|---|
| With C37H40Cl2N2ORuS2 In tetrahydrofuran at 22℃; for 1h; Alkene (Olefin) Metathesis; Glovebox; Inert atmosphere; stereoselective reaction; | 95% |
-
-
590-18-1
(Z)-2-Butene

-
-
3315-16-0
silver cyanate

-
-
19190-92-2, 19190-93-3, 89416-61-5
methyl N-threo-3-iodo-2-butylcarbamate

| Conditions | Yield |
|---|---|
| With iodine In diethyl ether 1.) 0 deg C, 2 h, 2.) room temperature, 1 h; | 94% |
| With iodine In tetrahydrofuran | |
| With iodine In diethyl ether |
-
-
590-18-1
(Z)-2-Butene

- poly[1-(11-allyloxy-undecylsulfanylmethyl)-4-vinyl benzene-co-vinyl-benzene-co-divinyl-benzene], 2 percent divinyl-benzene cross-links, high loading of side chains
-
poly[1-(11-allyloxy-undecylsulfanylmethyl)-4-vinyl benzene-co-vinyl-benzene-co-divinyl-benzene], 2 percent divinyl-benzene cross-links, high loading of side chains

- poly[(1-(11-((but-2-enyl)oxy)undecyl sulfanyl methyl)-4-vinyl benzene)-co-vinyl-benzene-co-divinyl-benzene], 2 percent divinyl-benzene cross-links, high loading of side chains
-
poly[(1-(11-((but-2-enyl)oxy)undecyl sulfanyl methyl)-4-vinyl benzene)-co-vinyl-benzene-co-divinyl-benzene], 2 percent divinyl-benzene cross-links, high loading of side chains

| Conditions | Yield |
|---|---|
| With Grubbs catalyst first generation In dichloromethane at 20℃; for 84h; | 94% |

| Conditions | Yield |
|---|---|
| in dark at -78°C (3 d); | 94% |
| in dark at -78°C (3 d); | 94% |
cis-2-Butene Specification
The cas register number of cis-2-Butene is 590-18-1. It also can be called as cis-1,2-Dimethylethylene and the IUPAC Name about this chemical is (Z)-but-2-ene. It belongs to the following product categories, such as Gas Cylinders, Hydrocarbons (Low Boiling point), Synthetic Organic Chemistry, Chemical Synthesis, Compressed and Liquefied Gases, Synthetic Reagents and so on.
Physical properties about cis-2-Butene are: (1)ACD/LogP: 2.34; (2)ACD/LogD (pH 5.5): 2.336; (3)ACD/LogD (pH 7.4): 2.336; (4)ACD/BCF (pH 5.5): 35.094; (5)ACD/BCF (pH 7.4): 35.094; (6)ACD/KOC (pH 5.5): 444.321; (7)ACD/KOC (pH 7.4): 444.321; (8)Index of Refraction: 1.384; (9)Molar Refractivity: 20.638 cm3; (10)Molar Volume: 88.177 cm3; (11)Polarizability: 8.182x10-24cm3; (12)Surface Tension: 16.795 dyne/cm; (13)Enthalpy of Vaporization: 23.34 kJ/mol; (14)Boiling Point: 3.719 °C at 760 mmHg; (15)Vapour Pressure: 1595.904 mmHg at 25°C.
Preparation: this chemical can be prepared by ethene, this reaction also can produce butane. This reaction will need reagent H2, ZrO2. The reaction reaction temperature is 298 ℃. The yield is about 48.9%.
![]()
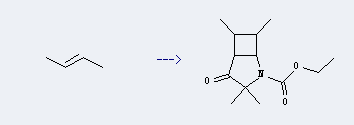
Uses of cis-2-Butene: it can be used to produce ethyl perhydro-2,2,4,5-tetramethyl-3-oxo-1H-cyclobuta[b]pyrrole-1-carboxylate. This reaction will need solvent acetonitrile with reaction time of 16 hours. The yield is about 78%.
When you are using this chemical, please be cautious about it as the following:
This chemical is extremely flammable. When you are using it, please keep container in a well-ventilated place and keep away from sources of ignition, you also need take precautionary measures against static discharges.
You can still convert the following datas into molecular structure:
(1)SMILES: C/C=C\C
(2)InChI: InChI=1/C4H8/c1-3-4-2/h3-4H,1-2H3/b4-3-
(3)InChIKey: IAQRGUVFOMOMEM-ARJAWSKDBO
(4)Std. InChI: InChI=1S/C4H8/c1-3-4-2/h3-4H,1-2H3/b4-3-
(5)Std. InChIKey: IAQRGUVFOMOMEM-ARJAWSKDSA-N
Related Products
- cis-2-Butene
- cis-2-Butenenitrile
- 590-19-2
- 59020-09-6
- 59020-10-9
- 59020-44-9
- 59020-85-8
- 59020-90-5
- 59021-02-2
- 59022-46-7
- 59025-03-5
- 5902-51-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View