-
Name
Fluorobenzene
- EINECS 207-321-7
- CAS No. 462-06-6
- Article Data381
- CAS DataBase
- Density 1.026 g/cm3
- Solubility insoluble in water
- Melting Point -42 °C
- Formula C6H5F
- Boiling Point 84.7 °C at 760 mmHg
- Molecular Weight 96.1041
- Flash Point -15 °C
- Transport Information UN 2387 3/PG 2
- Appearance Colorless liquid
- Safety 16-26-36-7-33-29-45-36/37-61-7/9
- Risk Codes 36/37/38-11-39/23/24/25-23/24/25-52/53-36
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi,  F,
F,  T
T
- Synonyms Monofluorobenzene;NSC 68416;Phenyl fluoride;Fluorbenzene;
- PSA 0.00000
- LogP 1.82570
Synthetic route

-
-
12775-96-1, 15158-11-9, 15721-63-8, 16941-75-6, 17493-86-6, 19498-52-3, 20499-83-6, 20499-84-7, 20499-85-8, 20499-86-9, 20573-10-8, 20573-11-9, 21595-51-7, 21595-52-8, 22206-52-6, 26445-28-3, 28959-95-7, 37362-93-9, 39417-05-5, 54603-16-6, 54603-23-5, 54603-32-6, 54603-40-6, 54603-48-4, 54603-81-5, 54603-89-3, 56316-56-4, 95985-91-4, 122297-32-9, 7440-50-8
copper

-
A
-
462-06-6
fluorobenzene

-
B
-
591-50-4
iodobenzene

-
C
-
92-52-4
biphenyl

-
D
-
14708-11-3
copper(I) tetrafluoroborate

-
E
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| In acetonitrile Kinetics; stirred at 20-70°C; GLC; | A <1 B 100% C 95% D 99% E 5% |

-
-
99506-39-5
4-fluorophenyl(m-carboran-9-yl)iodonium tetrafluoroborate

-
-
7647-14-5
sodium chloride

-
A
-
462-06-6
fluorobenzene

-
B
-
17157-02-7
9-iodo-m-carborane

-
C
-
17819-85-1
9-chloro-m-carborane

-
D
-
352-34-1
4-fluoro-1-iodobenzene

| Conditions | Yield |
|---|---|
| In chloroform; water mixt. of aryl(m-carboran-9-yl)iodonium tetrafluoroborate, NaCl, water and chloroform was vigorously stirred under reflux at 56°C, 2-2.5 h; internal standard (chlorobenzene) added and org. layer was analysed by GLC; | A 0% B 0% C 100% D 100% |

-
-
3038-48-0
2-(trifluoromethyl)phenylacetic acid

-
-
214360-58-4
2-(4-fluorophenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane

-
A
-
462-06-6
fluorobenzene

| Conditions | Yield |
|---|---|
| With t-Boc-L-valine; 2,5-di-tert-butyl-p-benzoquinone; oxygen; potassium hydrogencarbonate In tert-Amyl alcohol at 90℃; under 2280.15 Torr; for 24h; Catalytic behavior; | A n/a B 99% |


| Conditions | Yield |
|---|---|
| Stage #1: aniline With hydrogen fluoride at -10 - 0.5℃; Flow reactor; Large scale; Stage #2: With nitrosylsulfuric acid at 0 - 10℃; Temperature; Flow reactor; Large scale; | 98.9% |
| With pyridine; hydrogen fluoride; sodium nitrite Product distribution; changed molar ratio HF/Pyr, changed time and temperature, only HF; multistep reaction; 1.) 20 deg C, 30 min., 2.) 55 deg C, 1 h; | 95% |
| Stage #1: aniline With hydrogenchloride; fluoroboric acid; sodium nitrite In water at 25℃; for 0.00416667h; Balz-Schiemann Reaction; Stage #2: at 125℃; for 0.0166667h; Balz-Schiemann Reaction; | 92% |
-
-
446-46-8
2-fluorobenzenediazonium tetrafluoroborate

-
A
-
462-06-6
fluorobenzene

-
B
-
367-11-3
ortho-difluorobenzene

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate; hydrogen fluoride at 12℃; for 1h; Decomposition; Fluoro-dediazoniation; Irradiation; | A 0.2% B 98.9% |
| With boron trifluoride diethyl etherate at 12℃; for 3h; Decomposition; Fluoro-dediazoniation; Irradiation; | A 10.7% B 84.6% |

| Conditions | Yield |
|---|---|
| With C60H48BP3Pd; potassium formate; [2.2.2]cryptande In tetrahydrofuran at 60℃; for 72h; Schlenk technique; Inert atmosphere; | 97% |

| Conditions | Yield |
|---|---|
| With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; cesium fluoride In 2-pentanol at 100℃; for 18h; Inert atmosphere; | A 95.5% B 4.5% |
| With palladium; hydroquinone; potassium hydroxide In glycerol at 90℃; for 18h; chemoselective reaction; | A n/a B 71 %Chromat. |

| Conditions | Yield |
|---|---|
| With pyridine; hydrogen fluoride at 55℃; for 1h; Product distribution; Rate constant; Thermodynamic data; changed molar ratio HF/Pyr, temp. and time; energy/enthalpy of activation; | 95% |
| With tetrakis(acetonitrile)copper(I)tetrafluoroborate; [4,4′-bis(1,1-dimethylethyl)-2,2′-bipyridine-N1,N1′]bis{3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl-κN]phenyl-κC}iridium(III) hexafluorophosphate; cesium fluoride In acetone at 30℃; Irradiation; | 37% |
| With sodium difluorohydrogenate; 1,2,4-Trichlorobenzene |

| Conditions | Yield |
|---|---|
| With fluorine at 60℃; under 3750.38 Torr; Temperature; | 95% |
| With trifluorormethanesulfonic acid; Selectfluor In dichloromethane at 40℃; for 20h; Fluorination; | 83% |
| With Al2CuF8 at 500℃; Reagent/catalyst; Inert atmosphere; | 47% |

| Conditions | Yield |
|---|---|
| With formaldehyd; palladium diacetate; caesium carbonate In dimethyl sulfoxide at 80℃; for 12h; | 94% |
| With sodium tetrahydroborate; di-tert-butyl peroxide In N,N-dimethyl-formamide at 70℃; for 2h; Irradiation; | 82 % Chromat. |
| With hydrogenchloride; CuI*P(Et)3; naphthalen-1-yl-lithium 1.) THF, 25 deg C, 10 min; Yield given. Multistep reaction; |

-
A
-
462-06-6
fluorobenzene

-
B
-
28625-02-7
thallium(I) tetrafluoroborate

| Conditions | Yield |
|---|---|
| In solid byproducts: BF3; pyrolisis under Ar without solvent at 100-150°; TlBF4 identified by IR spectrum and comparison of m.p. with that of an authentic sample; | A 51% B 94% |

| Conditions | Yield |
|---|---|
| With quinoline; copper(I) oxide; 1,10-Phenanthroline; tetradecane In 1-methyl-pyrrolidin-2-one at 170℃; for 6h; Reagent/catalyst; Inert atmosphere; | 91% |
| copper(I) oxide; 1,10-Phenanthroline In quinoline at 170℃; for 6h; | 75% |
| With [Rh(OH)(cod)]2; 1,3-bis-(diphenylphosphino)propane; water; sodium hydroxide In tetrahydrofuran at 150℃; for 24h; Inert atmosphere; | 8 %Chromat. |
-
-
352-34-1
4-fluoro-1-iodobenzene

-
-
201230-82-2
carbon monoxide

-
A
-
462-06-6
fluorobenzene

-
B
-
459-57-4
4-fluorobenzaldehyde

| Conditions | Yield |
|---|---|
| With hydrogen; potassium carbonate In 1,4-dioxane at 120 - 140℃; under 30003 Torr; for 20h; Autoclave; | A 7% B 91% |

-
-
99506-39-5
4-fluorophenyl(m-carboran-9-yl)iodonium tetrafluoroborate

-
A
-
462-06-6
fluorobenzene

-
B
-
17157-02-7
9-iodo-m-carborane

-
C
-
54360-42-8
9-hydroxy-1,7-dicarba-closo-dodecaborane

-
D
-
114992-96-0
9-nitro-m-carbaborane

-
E
-
352-34-1
4-fluoro-1-iodobenzene

| Conditions | Yield |
|---|---|
| With sodium nitrite In dichloromethane; water mixt. of NaNO2 and the boron contg. salt in a two phase system stirred vigorously at 40°C, 2.5h (accompanied by evolution of nitrogen oxides), further product: 4-F-C6H4NO2 (traces); organic layer sepd. from aq. layer, evapn. of CH2Cl2 in vac., residue sepd. by chromy. (silica gel, hexane (9-iodo-m-carbaborane); benzene-hexane 1:1 (9-nitro-m-carbaborane); ether (9-hydroxy-m-carbaborane); | A 5% B 8% C 75% D 8% E 90% |


-
-
462-06-6
fluorobenzene

| Conditions | Yield |
|---|---|
| With Selectfluor chemoselective reaction; | 90% |
-
-
128812-96-4
perfluoro-

-
-
71-43-2
benzene

-
A
-
462-06-6
fluorobenzene

-
B
-
128812-95-3
1,1,1-trifluoro-N-(perfluoropyridin-4-yl)methanesulfonamide

| Conditions | Yield |
|---|---|
| In chloroform-d1 at 60℃; for 0.166667h; sealed in vacuo; | A 88% B n/a |


| Conditions | Yield |
|---|---|
| With 1,3-bis(2,6-diisopropylphenyl)-2,2-difluoro-2,3-dihydro-1h-imidazole; cesium fluoride In toluene at 80℃; for 18h; Product distribution / selectivity; Sealed vial; | 88% |
| With 1,3-bis(2,6-diisopropylphenyl)-2,2-difluoro-2,3-dihydro-1h-imidazole; cesium fluoride In toluene at 23 - 80℃; for 18.5h; Inert atmosphere; | 82 %Spectr. |
| Multi-step reaction with 2 steps 1: fluorosulfonyl fluoride; triethylamine / 1,4-dioxane / 24 h / 20 °C 2: tetramethylammonium fluoride / N,N-dimethyl-formamide / 24 h / 100 °C / Inert atmosphere; Glovebox; Sealed tube View Scheme |

| Conditions | Yield |
|---|---|
| With potassium trimethylsilonate In dimethyl sulfoxide at 60℃; under 760.051 Torr; for 5.5h; Catalytic behavior; Reagent/catalyst; Solvent; Sealed tube; | 88% |
| With potassium trimethylsilonate In dimethyl sulfoxide at 70℃; for 6h; Sealed tube; Schlenk technique; | 85 %Chromat. |

| Conditions | Yield |
|---|---|
| With pyridine; hydrogen fluoride for 2h; Decomposition; Fluoro-dediazoniation; Irradiation; | A 87.9% B 0.4% |

-
-
462-06-6
fluorobenzene

| Conditions | Yield |
|---|---|
| With potassium fluoride at 160 - 170℃; for 20h; | 85% |

| Conditions | Yield |
|---|---|
| With potassium fluoride; 18-crown-6 ether; copper(II) methanesulfonate In N,N-dimethyl-formamide at 60℃; for 18h; Reagent/catalyst; Time; Solvent; | A 85% B n/a |
| With potassium fluoride; 18-crown-6 ether; copper(II) bis(trifluoromethanesulfonate) In ethyl acetate at 60℃; for 18h; Reagent/catalyst; Solvent; Glovebox; Overall yield = 39 %Spectr.; | |
| With tetrabutyl ammonium fluoride; copper(II) bis(trifluoromethanesulfonate) In N,N-dimethyl-formamide at 60℃; for 18h; Glovebox; |

-
-
462-06-6
fluorobenzene

| Conditions | Yield |
|---|---|
| With potassium fluoride; 18-crown-6 ether; copper(II) bis(trifluoromethanesulfonate) In N,N-dimethyl-formamide at 60℃; for 18h; Mechanism; Reagent/catalyst; Solvent; Time; Glovebox; | 85% |
| With tetrabutyl ammonium fluoride; copper(II) bis(trifluoromethanesulfonate) In N,N-dimethyl-formamide at 60℃; for 18h; Glovebox; | 29% |
-
-
54416-55-6
tris(4-fluorophenyl)cyanurate

-
-
462-06-6
fluorobenzene

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)nickel (0); sodium isopropylate; 1,3-bis[(2,6-diisopropyl)phenyl]imidazolinium chloride In 2-methyltetrahydrofuran at 80℃; for 12h; Green chemistry; | 85% |

| Conditions | Yield |
|---|---|
| With sodium carbonate In N,N-dimethyl acetamide at 100℃; for 7h; Schlenk technique; Inert atmosphere; | A 13% B 85% |


| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran for 8h; Heating; | 84% |
| Multi-step reaction with 3 steps 1: potassium iodide / water; acetonitrile 2: silver(l) oxide / water / 11 h / 0 - 20 °C 3: dimethylsulfoxide-d6 / 0.17 h / 100 °C View Scheme |

| Conditions | Yield |
|---|---|
| With hypofluorous acid trifluoromethyl ester In chloroform for 0.5h; Ambient temperature; | 83% |
| With sulfur tetrafluoride at -70 - -60℃; for 8h; | 58% |
-
-
768-32-1
trimethylphenylsilane

-
-
546-67-8
lead(IV) tetraacetate

-
-
109-63-7
trifluoroborane diethyl ether

-
A
-
462-06-6
fluorobenzene

-
B
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| Stirring of silane with a slurry of Pb(OAc)4 in excess of BF3*Et2O overnight at room temp.; GC anal.; | A 83% B 6% |

-
-
99506-39-5
4-fluorophenyl(m-carboran-9-yl)iodonium tetrafluoroborate

-
A
-
462-06-6
fluorobenzene

-
B
-
17157-02-7
9-iodo-m-carborane

-
C
-
73050-37-0
9-fluoro-m-carborane

-
D
-
352-34-1
4-fluoro-1-iodobenzene

| Conditions | Yield |
|---|---|
| In chloroform; water mixt. of aryl(m-carboran-9-yl)iodonium tetrafluoroborate, NaF, water and chloroform was vigorously stirred under reflux at 56°C, 1.25 h; internal standard (chlorobenzene) added and org. layer was analysed by GLC; | A 9% B 2% C 83% D 82% |


| Conditions | Yield |
|---|---|
| Stage #1: phenylboronic acid With sodium hydroxide In methanol at 23℃; for 0.25h; Inert atmosphere; Stage #2: With silver trifluoromethanesulfonate In methanol at 0℃; for 0.5h; Inert atmosphere; Stage #3: With Selectfluor In acetone at 23℃; for 1h; Inert atmosphere; Molecular sieve; regiospecific reaction; | 82% |
| Stage #1: phenylboronic acid With sodium hydroxide In methanol at 23℃; Stage #2: With silver trifluoromethanesulfonate In methanol at 0℃; for 0.5h; Stage #3: With Selectfluor In acetone for 0.5h; Molecular sieve; | |
| Stage #1: phenylboronic acid With sodium hydroxide In methanol at 23℃; for 0.25h; Stage #2: With silver trifluoromethanesulfonate In methanol at 0℃; for 0.5h; Stage #3: With Selectfluor In [(2)H6]acetone for 1h; Molecular sieve; | 95 %Spectr. |

-
-
462-06-6
fluorobenzene

| Conditions | Yield |
|---|---|
| With Selectfluor In [(2)H6]acetone at 50℃; for 0.166667h; Product distribution / selectivity; | 82% |
| With Selectfluor In [(2)H6]acetone at 50℃; for 0.166667h; Product distribution / selectivity; | 47% |
fluorobenzene Chemical Properties
Product Name:Fluorobenzene(462-06-6)
CAS No:462-06-6
MF: C6H5F
MW: 96.1
mp : -42 °C
bp : 85 °C(lit.)
density : 1.024 g/mL at 25 °C(lit.)
vapor density : 3.31 (vs air)
refractive index : n20/D 1.465(lit.)
Fp : 9 °F
storage temp. : 0-6°C
Water Solubility : Insoluble
Synonyms: benzene,benzene,fluoro-;fluoro-benzen;Fluorobenzenes;Fluorylfluoride;Monofluorobenzene;Phenyl fluoride;Phenylfluoride;FLUOROBENZENE
Molecular Structure: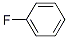
General Description of Fluorobenzene(462-06-6): A clear, colorless liquid with a characteristic aromatic odor. About the same density as water. Flash point 5°F. Vapors heavier than air. May irritate the skin, eyes, and mucous membranes. Used as an insecticide, larvacide and as a reagent for plastic or resin polymers.
fluorobenzene Production
On the laboratory scale, PhF is conveniently prepared by the thermal decomposition of the phenyldiazonium tetrafluoroborate:
PhN2BF4 → PhF + BF3 + N2
According to the procedure, solid [PhN2]BF4 is heated with a flame to initiate an exothermic reaction that affords two volatile products, PhF and BF3, which are readily separated because of their differing boiling points.PhF was first reported in 1886 by O. Wallach at the University of Bonn, who prepared the compound in two steps, starting also with a phenyldiazonium salt. The diazonium chloride was first converted to its piperidinide, which in turn was cleaved using hydrofluoric acid.
[PhN2]Cl + 2 C5H10NH → PhN=N-NC5H10 + [C5H10NH2]Cl
PhN=N-NC5H10 + 2 HF → PhF + N2 + [C5H10NH2]F
An interesting historical note: in Wallach’s era, the element fluorine was symbolized with “Fl”. Thus, his procedure is subtitled “Fluorbenzol(462-06-6), C6H5Fl”.The technical synthesis is by the reaction of cyclopentadiene with difluorocarbene. The initially formed cyclopropane undergoes a ring expansion and subsequent elimination of hydrogen fluoride.
fluorobenzene Toxicity Data With Reference
LD50/LC50: RTECS:
CAS# 462-06-6: Inhalation, mouse: LC50 = 45 gm/m3/2H;
Inhalation, mouse: LC50 = 45000 mg/m3/2H;
Inhalation, rat: LC50 = 26908 mg/m3;
Oral, rat: LD50 = 4399 mg/kg;
Carcinogenicity: Fluorobenzene - Not listed as a carcinogen by ACGIH, IARC, NTP, or CA Prop 65.
Other: See actual entry in RTECS for complete information.
fluorobenzene Safety Profile
Risk Statements : 36/37/38-11-39/23/24/25-23/24/25-52/53-36
Safety Statements : 16-26-36-7-33-29-45-36/37-61
RIDADR : UN 2387 3/PG 2
WGK Germany : 2
Hazard Note : Flammable
TSCA : T
HazardClass : 3
PackingGroup : II
fluorobenzene Specification
European Labeling in Accordance with EC Directives
Hazard Symbols: F
Risk Phrases:
R 11 Highly flammable.
Safety Phrases:
S 7 Keep container tightly closed.
S 16 Keep away from sources of ignition - No smoking.
S 29 Do not empty into drains.
S 33 Take precautionary measures against static discharges.
WGK (Water Danger/Protection)
CAS# 462-06-6: 2
Canada
CAS# 462-06-6 is listed on Canada's DSL List
US Federal
TSCA
CAS# 462-06-6 is listed on the TSCA Inventory.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View