-
Name
3-Methoxybenzaldehyde
- EINECS 209-712-8
- CAS No. 591-31-1
- Article Data374
- CAS DataBase
- Density 1.088 g/cm3
- Solubility Soluble in alcohol, ether and benzene, insoluble in water
- Melting Point 187 °C
- Formula C8H8O2
- Boiling Point 230.761 °C at 760 mmHg
- Molecular Weight 136.15
- Flash Point 100.219 °C
- Transport Information
- Appearance clear pale yellow to yellow liquid
- Safety 26-36-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 3-Methoxy benzaldehyde;Metamethoxybenzaldehyde;4-08-00-00241 (Beilstein Handbook Reference);3-Anisaldehyde;m-Methoxybenzaldehyde;
- PSA 26.30000
- LogP 1.50770
Synthetic route

| Conditions | Yield |
|---|---|
| With oxygen; perruthenate modified mesoporous silicate MCM-41 In toluene at 80℃; for 3h; Oxidation; | 100% |
| With Oxone; [Mn(NO3)2(2,3,5,6-tetra(2-pyridyl)pyrazine)(H2O)]; tetrabutylammomium bromide In dichloromethane at 20℃; Catalytic behavior; Reagent/catalyst; | 100% |
| With 1-benzyl-1-aza-4-azoniabicyclo<2.2.2>octane periodate In acetonitrile for 10h; Heating; | 99% |
-
-
59184-17-7
1,1-diacetoxy-1-(3-methoxyphenyl)methane

-
-
591-31-1
3-methoxy-benzaldehyde

| Conditions | Yield |
|---|---|
| With sulphated zirconia In acetonitrile at 60℃; for 1h; Microwave irradiation; | 100% |
| With (NH4)3PW12O40 In methanol at 20℃; for 1h; | 93% |
| With poly(4-vinylpyridinium) hydrogen sulfate solid acid In methanol at 20℃; for 0.966667h; Irradiation; | 92% |

| Conditions | Yield |
|---|---|
| With polymethylhydrosiloxane; trifuran-2-yl-phosphane; tetrabutyl ammonium fluoride; potassium fluoride; tris-(dibenzylideneacetone)dipalladium(0) In tetrahydrofuran; water at 20℃; for 1h; | 98% |
| With palladium; xylene at 140 - 150℃; Hydrogenation; | |
| With sodium tris(tert-butoxo)aluminium hydride In tetrahydrofuran; diethylene glycol dimethyl ether at -78℃; for 3h; Yield given; | |
| Stage #1: m-anisoyl chloride With aluminium hydride In tetrahydrofuran at 20℃; for 1h; Metallation; Stage #2: With pyridinium chlorochromate In tetrahydrofuran; dichloromethane at 20℃; for 3h; Oxidation; | 97 % Chromat. |
-
-
132816-03-6
(3-Methoxy-benzyloxy)-trimethyl-silane

-
-
591-31-1
3-methoxy-benzaldehyde

| Conditions | Yield |
|---|---|
| With aluminium trichloride; tetramethylammonium chlorochromate In acetonitrile for 1.08333h; Heating; | 98% |
| With n-butyltriphenylphosphonium peroxodisulfate In acetonitrile for 0.166667h; Heating; | 96% |
| With aluminium trichloride; benzyltriphenylphosphonium chlorochromate In acetonitrile for 0.75h; Heating; | 95% |

-
-
591-31-1
3-methoxy-benzaldehyde

| Conditions | Yield |
|---|---|
| With benzyltriphenylphosphonium peroxodisulfate In acetonitrile for 0.1h; Heating; | 98% |
| With quinolinium monofluorochromate(VI) In dichloromethane at 20℃; for 0.8h; | 93% |
| With cetyltrimethylammonium peroxodisulphate In acetonitrile for 0.333333h; Reflux; | 93% |

| Conditions | Yield |
|---|---|
| With water; sodium hydroxide at 20℃; for 0.05h; Microwave irradiation; | 98% |
| With 4-methylmorpholine N-oxide; 1-ethyl-3-methyl-1H-imidazol-3-ium chloride; potassium iodide at 100℃; for 0.0333333h; Microwave irradiation; Ionic liquid; | 92% |
| With sodium nitrate; acetic acid In water for 7.5h; Reflux; | 81% |
-
-
82632-37-9
N-tert-butyl-1-(3-methoxyphenyl)methanimine

-
-
591-31-1
3-methoxy-benzaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: N-tert-butyl-1-(3-methoxyphenyl)methanimine With chromium dichloride In tetrahydrofuran at 25℃; for 0.0833333h; Schlenk technique; Inert atmosphere; Stage #2: With phenylmagnesium bromide In tetrahydrofuran at 25℃; for 5h; Schlenk technique; Inert atmosphere; Stage #3: With hydrogenchloride In tetrahydrofuran; water at 25℃; for 3h; Schlenk technique; Inert atmosphere; regioselective reaction; | 98% |
| With water |
-
-
2398-37-0
3-methoxyphenyl bromide

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
591-31-1
3-methoxy-benzaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: 3-methoxyphenyl bromide With n-butyllithium; isopropylmagnesium chloride In tetrahydrofuran; hexane at 0 - 5℃; for 1h; Stage #2: N,N-dimethyl-formamide In tetrahydrofuran at 0℃; for 1h; Further stages.; | 96% |
| Ambient temperature; Mg anode, Cd coated catode, Bu4NBr electrolyte; | 56% |
| Stage #1: 3-methoxyphenyl bromide With n-butyllithium In tetrahydrofuran; hexane at 0℃; Stage #2: N,N-dimethyl-formamide In tetrahydrofuran; hexane at 0℃; |
-
-
19693-78-8
2-(3-methoxyphenyl)-1,3-dioxolane

-
-
591-31-1
3-methoxy-benzaldehyde

| Conditions | Yield |
|---|---|
| With 1-benzyl-4-aza-1-azoniabicyclo[2.2.2]octane dichromate In dichloromethane for 0.05h; Irradiation; | 96% |
| With aluminium trichloride; 1-benzyl-4-aza-1-azoniabicyclo[2.2.2]octane dichromate for 0.00833333h; | 96% |
| With 2,6-dicarboxypyridinium chlorochromate In acetonitrile at 20℃; for 0.25h; | 94% |
| With potassium dichromate; aluminium trichloride for 0.0833333h; | 90% |
| With K5 In acetone for 0.166667h; Heating; | 97 % Chromat. |

| Conditions | Yield |
|---|---|
| With pyridine; 1,3-bis-(diphenylphosphino)propane; hydrogen; palladium diacetate In dimethyl sulfoxide at 120℃; under 7500.75 Torr; for 2h; Flow reactor; | 96% |
-
-
38489-80-4
3-methoxybenzaldehyde oxime

-
-
591-31-1
3-methoxy-benzaldehyde

| Conditions | Yield |
|---|---|
| With aluminium trichloride; benzyltriphenylphosphonium chlorochromate In acetonitrile for 0.666667h; Heating; | 95% |
| With potassium permanganate; 1-n-butyl-3-methylimidazolim bromide at 20℃; for 0.7h; Ionic liquid; chemoselective reaction; | 94% |
| With bismuth(III) chloride; benzyltriphenylphosphonium peroxymonosulfate In acetonitrile for 0.666667h; Heating; | 90% |

-
-
591-31-1
3-methoxy-benzaldehyde

| Conditions | Yield |
|---|---|
| With NTPPPODS In water; acetonitrile for 0.25h; Reflux; | 95% |
| With benzyltriphenylphosphonium peroxodisulfate In acetonitrile for 0.166667h; Heating; | 94% |
| With quinolinium monofluorochromate(VI) In acetonitrile for 3h; Heating; | 93% |
-
-
17637-72-8
(E)-1-(3-methoxyphenyl)-N-phenylmethanimine

-
-
591-31-1
3-methoxy-benzaldehyde

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water In diethyl ether; toluene at 20℃; for 2h; Inert atmosphere; | 95% |
-
-
1448723-38-3
C15H16N2O4S

-
-
591-31-1
3-methoxy-benzaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: C15H16N2O4S With 1H-imidazole; 1-(Trimethylsilyl)imidazole In toluene at 55℃; McFadyen-Stevens Reaction; Stage #2: With citric acid In methanol at 20℃; McFadyen-Stevens Reaction; | 95% |
| Stage #1: C15H16N2O4S With 1H-imidazole; 1-(Trimethylsilyl)imidazole In toluene at 55℃; Inert atmosphere; Stage #2: With citric acid In toluene at 23℃; Inert atmosphere; | 95% |
-
-
121336-27-4
1-Methoxy-3-phenethyloxymethyl-benzene

-
A
-
60-12-8
2-phenylethanol

-
B
-
591-31-1
3-methoxy-benzaldehyde

| Conditions | Yield |
|---|---|
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In dichloromethane; water at 20℃; for 24h; | A 80% B 94% |
-
-
123767-44-2
3-methoxylbenzyl azide

-
-
591-31-1
3-methoxy-benzaldehyde

| Conditions | Yield |
|---|---|
| With iron(III) chloride; dihydrogen peroxide In dichloromethane; water for 13h; Reflux; Air; | 94% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 25 - 30℃; for 12h; Inert atmosphere; | 93% |
| With potassium carbonate In acetone for 3h; Reflux; | 92% |
| With potassium carbonate In N,N-dimethyl-formamide | 91% |

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide; sodium phosphate at 105℃; for 3h; Green chemistry; | 93% |
| With 1,4-diaza-bicyclo[2.2.2]octane; palladium diacetate; dicyclohexyl-carbodiimide at 100℃; for 2h; Sealed tube; | 85% |
| With iodine; triethylamine; triphenylphosphine In toluene at 80℃; for 2h; Sealed tube; | 80% |
| With iodine; triethylamine; triphenylphosphine In toluene at 80℃; Inert atmosphere; Sealed tube; | 79% |
-
-
54308-43-9
2-(3-methoxyphenyl)-1,3-dithiane

-
-
591-31-1
3-methoxy-benzaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: 2-(3-methoxyphenyl)-1,3-dithiane With trichloroisocyanuric acid; silica gel at 20℃; for 0.05h; Stage #2: With water at 20℃; | 92% |
| With p-benzoquinone; sodium iodide In water; acetonitrile at 100℃; for 24h; | 91% |
| With eosin y In water; acetonitrile at 20℃; for 2h; Irradiation; | 91% |
| With tetrachlorosilane; dimethyl sulfoxide In dichloromethane at 20℃; for 0.75h; | 90% |
| With water In 1,4-dioxane at 100℃; for 48h; Inert atmosphere; | 74% |
-
-
689247-71-0
1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-heptadecafluoro-octane-1-sulfonic acid 4-formyl-2-methoxy-phenyl ester

-
-
591-31-1
3-methoxy-benzaldehyde

| Conditions | Yield |
|---|---|
| With formic acid; potassium carbonate; (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride In water; acetone; toluene at 100℃; for 0.333333h; microwave irradiation; | 92% |

-
-
591-31-1
3-methoxy-benzaldehyde

| Conditions | Yield |
|---|---|
| With Iron(III) nitrate nonahydrate at 90℃; for 0.133333h; | 92% |
-
-
28145-59-7
ethyl 3-methoxythiobenzoate

-
-
591-31-1
3-methoxy-benzaldehyde

| Conditions | Yield |
|---|---|
| With triethylsilane; palladium on activated charcoal In acetone at 20℃; | 91% |
| With triethylsilane; palladium on activated charcoal In tetrahydrofuran at 20℃; for 5h; Fukuyama reduction; | 90% |
| With triethylsilane; palladium on activated charcoal In acetone Ambient temperature; Yield given; |

| Conditions | Yield |
|---|---|
| With eosin Y; oxygen In dimethyl sulfoxide at 20℃; for 9h; Irradiation; Green chemistry; | 91% |
| With iron(III) trifluoromethanesulfonate; 2-((4R,5R)-1-((4-(tert-butyl)phenyl)sulfonyl)-4,5-diphenylimidazolidin-2-yl)-6-((4R,5R)-1-((4-(tert-butyl)phenyl)sulfonyl)-4,5-diphenylimidazolidin-2-yl)pyridine; oxygen In 1,2-dichloro-ethane at 70℃; under 760.051 Torr; for 6h; Green chemistry; chemoselective reaction; | 78% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 0.0833333h; Etherification; methylation; microwave irradiation; | 90% |
| With sodium hydroxide | |
| With sodium hydroxide |
-
-
18483-97-1
2-(3-methoxybenzyloxy)tetrahydro-2H-pyran

-
-
591-31-1
3-methoxy-benzaldehyde

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; molybdenum trioxide In water; acetonitrile for 0.34h; Reflux; | 90% |
| With bismuth(III) chloride; benzyltriphenylphosphonium peroxymonosulfate In acetonitrile for 0.5h; Heating; | 88% |
| With aluminium trichloride; benzyltriphenylphosphonium chlorochromate In acetonitrile for 0.5h; Heating; | 88% |

-
-
591-31-1
3-methoxy-benzaldehyde

| Conditions | Yield |
|---|---|
| With Montmorillonite KSF clay for 0.00277778h; Elimination; microwave irradiation; | 90% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; oxygen In dimethyl sulfoxide at 120℃; for 26h; chemoselective reaction; | 90% |
-
-
6258-45-3
5-[(3-methoxyphenyl)methylene]-2,2-dimethyl-1,3-dioxane-4,6-dione

-
-
591-31-1
3-methoxy-benzaldehyde

| Conditions | Yield |
|---|---|
| With oxone In water; acetonitrile at 45℃; for 1h; | 90% |
-
-
127598-74-7
N-(2-cyanoethyl) N-(3-methoxybenzyl)-4-methylaniline

-
A
-
118072-35-8
3-(2-Dimethylaminomethyl-4-methyl-phenylamino)-propionitrile

-
B
-
591-31-1
3-methoxy-benzaldehyde

| Conditions | Yield |
|---|---|
| With water; trichlorophosphate In N,N-dimethyl-formamide Vilsmeier reaction; | A 80% B 89% |
-
-
24588-72-5
2-(4-methoxyphenyl)-1,3-dithiane

-
-
591-31-1
3-methoxy-benzaldehyde

| Conditions | Yield |
|---|---|
| With bismuth(III) chloride; benzyltriphenylphosphonium peroxymonosulfate In acetonitrile for 3h; Heating; | 89% |

| Conditions | Yield |
|---|---|
| at 20℃; for 12h; | 100% |
| In ethanol at 0 - 40℃; | 90% |
| In water for 10h; Ambient temperature; | 86% |

| Conditions | Yield |
|---|---|
| With hydrogen; Pd as electrode electrochemical reaction; | 100% |
| With sodium tetrahydroborate In methanol at 20℃; for 1h; | 100% |
| With sodium tetrahydroborate; Dowex1-x8 In tetrahydrofuran at 20℃; for 0.5h; | 99% |
-
-
867-13-0
diethoxyphosphoryl-acetic acid ethyl ester

-
-
591-31-1
3-methoxy-benzaldehyde

-
-
33877-04-2, 24393-55-3
ethyl (E)-3-(3-methoxyphenyl)acrylate

| Conditions | Yield |
|---|---|
| Stage #1: diethoxyphosphoryl-acetic acid ethyl ester With lithium chloride In tetrahydrofuran at 20℃; for 0.25h; Horner-Wadsworth-Emmons Olefination; Inert atmosphere; Stage #2: 3-methoxy-benzaldehyde With 1,8-diazabicyclo[5.4.0]undec-7-ene In tetrahydrofuran Horner-Wadsworth-Emmons Olefination; Inert atmosphere; | 100% |
| With sodium hydride In tetrahydrofuran; mineral oil at 0 - 20℃; for 1h; | 100% |
| With tetrabutyl ammonium fluoride In tetrahydrofuran at 30℃; for 4h; Glovebox; | 98% |
-
-
2398-37-0
3-methoxyphenyl bromide

-
-
591-31-1
3-methoxy-benzaldehyde

-
-
55360-45-7
bis(3-methoxyphenyl)methanol

| Conditions | Yield |
|---|---|
| With n-butyllithium In hexane; ethyl acetate | 100% |
| With magnesium In diethyl ether for 1h; Ambient temperature; | 62% |
| With magnesium 1.) ether, ultrasound; Yield given. Multistep reaction; | |
| Stage #1: 3-methoxyphenyl bromide With magnesium In tetrahydrofuran at 0℃; Reflux; Inert atmosphere; Stage #2: 3-methoxy-benzaldehyde In tetrahydrofuran at 0 - 20℃; Inert atmosphere; Stage #3: With hydrogenchloride; water In tetrahydrofuran | |
| Stage #1: 3-methoxyphenyl bromide With magnesium In diethyl ether at 20℃; for 2h; Inert atmosphere; Stage #2: 3-methoxy-benzaldehyde In diethyl ether at 20℃; for 2h; Inert atmosphere; |
-
-
558-13-4
carbon tetrabromide

-
-
591-31-1
3-methoxy-benzaldehyde

-
-
253684-23-0
1,1-dibromo-2-(3-methoxyphenyl)ethene

| Conditions | Yield |
|---|---|
| With triphenylphosphine In dichloromethane | 100% |
| With triphenylphosphine In dichloromethane at 0℃; for 5.25h; Inert atmosphere; | 96% |
| Stage #1: carbon tetrabromide With triphenylphosphine In dichloromethane at 0℃; for 0.5h; Inert atmosphere; Stage #2: 3-methoxy-benzaldehyde In dichloromethane at 0℃; for 1.08333h; Inert atmosphere; | 94% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-methoxy-benzaldehyde With hydrogenchloride; sodium sulfite In tetrahydrofuran; water at 20℃; for 0.166667h; Stage #2: potassium cyanide In tetrahydrofuran; water for 0.5h; | 100% |
| With sodium hydrogensulfite 1) H2O, 40 deg C, 0.5 h, 2) H2O, 0 deg C, 1 h; Multistep reaction; | |
| With acetic acid In water | |
| With hydrogenchloride; sodium sulfite In tetrahydrofuran at 20℃; for 0.5h; |
-
-
591-31-1
3-methoxy-benzaldehyde

-
-
67-64-1
acetone

-
-
20766-31-8, 30625-53-7
(E)-4-(3-methoxyphenyl)but-3-en-2-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 0 - 25℃; for 5h; | 100% |
| With 1-n-butyl-3-methylimidazolim bromide at 60℃; for 6h; Aldol condensation; Enzymatic reaction; | 87% |
| With sodium hydroxide for 1.66667h; | 83% |
-
-
591-31-1
3-methoxy-benzaldehyde

-
-
74-89-5
methylamine

-
-
41789-95-1
1-(3-methoxyphenyl)-N-methylmethanamine

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In methanol; water at 0℃; for 2h; | 100% |
| With sodium tetrahydroborate In methanol; water at 0 - 20℃; | 99% |
| Stage #1: 3-methoxy-benzaldehyde; methylamine In methanol; water at 0 - 20℃; for 15.3333h; Stage #2: With sodium tetrahydroborate In methanol; 20C at 0 - 20℃; for 2 - 3h; Product distribution / selectivity; | 29% |
-
-
591-31-1
3-methoxy-benzaldehyde

-
-
1576-35-8
toluene-4-sulfonic acid hydrazide

-
-
19350-71-1
N'-(3-methoxybenzylidene)-4-methylbenzen sulfonohydrazide

| Conditions | Yield |
|---|---|
| In ethanol for 8h; Reflux; | 100% |
| With polystyrene sulfonic acid In water at 100℃; for 0.0833333h; Wavelength; Reagent/catalyst; Solvent; Temperature; Microwave irradiation; Green chemistry; | 92% |
| In methanol at 20℃; for 3h; | 90% |
-
-
557-20-0
diethylzinc

-
-
591-31-1
3-methoxy-benzaldehyde

-
-
134677-28-4
(1S)-1-(3-methoxyphenyl)propan-1-ol

| Conditions | Yield |
|---|---|
| (1S)-1-(9-piperidylfluoren-9-yl)ethanol In hexane; toluene at 0℃; for 4h; Addition; | 100% |
| Stage #1: diethylzinc In toluene at 0℃; for 0.5h; Stage #2: 3-methoxy-benzaldehyde In toluene at 0 - 20℃; for 48h; Reagent/catalyst; enantioselective reaction; | 99% |
| With (3S,4S)-2,2-dimethyl-4-[N-(9'-phenylfluoren-9'-yl)amino]pentan-3-ol In hexane; toluene at 18℃; for 1h; Addition; | 97% |

| Conditions | Yield |
|---|---|
| With (R,R)-(2,4,6-Me3C6H2-CH=N-CH(Ph))2; (R)-(+)-3,3'-diphenyl-[1,1'-binaphthalene]-2,2'-diol In hexane; dichloromethane 1.) -78 deg C, 4 h, 2.) -20 deg, 1 h; | 100% |
| With (1R,2R)-N,N'-bis[(2,4,6-trimethylphenyl)methylene]-1,2-cyclohexane-diamine; (R)-(+)-3,3'-diphenyl-[1,1'-binaphthalene]-2,2'-diol In hexane; dichloromethane at -78 - -20℃; for 5h; | 100% |
| Stage #1: diethylzinc With titanium(IV) isopropylate; (1R,2R)-1,2-bis(3,5-dibromophenyl)ethane-1,2-diol In hexane; dichloromethane at 25℃; for 0.333333h; Inert atmosphere; Stage #2: 3-methoxy-benzaldehyde In hexane; dichloromethane at 0℃; for 24h; Reagent/catalyst; Inert atmosphere; | 53% |
-
-
591-31-1
3-methoxy-benzaldehyde

-
-
149-73-5
trimethyl orthoformate

-
-
59276-28-7
m-anisaldehyde dimethyl acetal

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 1h; Inert atmosphere; | 100% |
| With ytterbium(III) chloride hexahydrate In methanol at 20℃; for 18h; | 98% |
| With toluene-4-sulfonic acid In methanol at 20℃; for 1h; Inert atmosphere; Sealed tube; | 93% |
-
-
64715-85-1, 91298-74-7, 127180-88-5
(R)-2-methoxy-1-phenylethylamine

-
-
591-31-1
3-methoxy-benzaldehyde

| Conditions | Yield |
|---|---|
| In toluene Heating; | 100% |
-
-
591-31-1
3-methoxy-benzaldehyde

-
-
97-62-1
Ethyl isobutyrate

-
-
602304-70-1, 293769-53-6
ethyl 3-hydroxy-3-(3-methoxyphenyl)-2,2-dimethylpropanoate

| Conditions | Yield |
|---|---|
| Stage #1: Ethyl isobutyrate With lithium diisopropyl amide In tetrahydrofuran at -78℃; for 0.5h; Stage #2: 3-methoxy-benzaldehyde In tetrahydrofuran at -78℃; for 1.5h; | 100% |

| Conditions | Yield |
|---|---|
| With potassium cyanide; 18-crown-6 ether; chiral salen-based titanium In dichloromethane at -40℃; for 24h; | 100% |
| With 3,3'-di-naphthalen-2-yl-biphenyl-2,2'-diol; titanium(IV) isopropylate; quinindine; isopropyl alcohol In toluene at -20℃; for 7h; Strecker reaction; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 99% |
| With chiral [(salen)TiO]2 In dichloromethane at -40℃; for 17h; | 94% |
| With chiral bimetallic titanium(salen) In dichloromethane at -40℃; for 17h; | 94% |
| With potassium cyanide; 18-crown-6 ether; titanium(salen) complex In dichloromethane at -40℃; for 24h; |
-
-
1076-38-6
4-hydroxy[1]benzopyran-2-one

-
-
591-31-1
3-methoxy-benzaldehyde

-
-
10172-74-4
3,3'-((3-methoxyphenyl)methylene)bis(4-hydroxy-2H-chromen-2-one)

| Conditions | Yield |
|---|---|
| With 4-sulfophthalic acid In water at 80℃; for 0.333333h; Catalytic behavior; Green chemistry; | 100% |
| With piperidine In ethanol at 20℃; for 4h; | 98% |
| With 4-aminobenzene sulfonic acid In water at 80℃; for 0.366667h; Green chemistry; | 96% |
-
-
591-31-1
3-methoxy-benzaldehyde

-
-
107-11-9
1-amino-2-propene

-
-
246509-60-4
N-[(3-methoxyphenyl)methylidene]-N-(2-propenyl)amine

| Conditions | Yield |
|---|---|
| With magnesium sulfate In dichloromethane at 20℃; for 20h; | 100% |
| With magnesium sulfate In dichloromethane for 1h; Heating; | 95% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium sulfite In tetrahydrofuran; water at 20℃; for 0.666667h; | 100% |
-
-
135636-66-7
butane-2,3-dione mono-oxime

-
-
591-31-1
3-methoxy-benzaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: butane-2,3-dione mono-oxime; 3-methoxy-benzaldehyde With hydrogenchloride; acetic acid In toluene at 22℃; for 16h; Stage #2: With sodium hydroxide In water; toluene at 32℃; pH=10.6; | 100% |
-
-
1005794-49-9
tert-butyl 1-(2-methylallyl)hydrazinecarboxylate

-
-
591-31-1
3-methoxy-benzaldehyde

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; | 100% |
-
-
75-64-9
tert-butylamine

-
-
591-31-1
3-methoxy-benzaldehyde

-
-
82632-37-9
N-tert-butyl-1-(3-methoxyphenyl)methanimine

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 4h; | 100% |
| With titanium tetrachloride In diethyl ether; toluene at 0℃; for 0.25h; Glovebox; Inert atmosphere; Schlenk technique; | 93% |
| at 100℃; for 4h; | 86% |
-
-
120943-70-6
dimethyl <2-(tert-butyldimethylsilyl)-2-oxoethyl>-phosphonate

-
-
591-31-1
3-methoxy-benzaldehyde

-
-
1204324-78-6
(E)-1-(tert-butyldimethylsilyl)-3-(3-methoxyphenyl)prop-2-en-1-one

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl <2-(tert-butyldimethylsilyl)-2-oxoethyl>-phosphonate With sodium hydride In tetrahydrofuran at 0 - 20℃; Horner-Wadsworth-Emmons olefination; Inert atmosphere; Stage #2: 3-methoxy-benzaldehyde In tetrahydrofuran at 20℃; for 15h; Horner-Wadsworth-Emmons olefination; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: trimethylsilyl cyanide; 3-methoxy-benzaldehyde; (S,S,S)-[Ru(2,2'-bis(diphenylphosphanyl)-1,1'-binaphthyl)bis(phenylglycinate)] In tetrahydrofuran; tert-butyl methyl ether at -78℃; for 0.166667h; Inert atmosphere; Stage #2: lithium chloride In tetrahydrofuran; tert-butyl methyl ether at -78℃; for 3h; Product distribution / selectivity; Inert atmosphere; | 100% |
-
-
591-31-1
3-methoxy-benzaldehyde

-
-
619-45-4
4-methoxycarbonyl aniline

-
-
349456-93-5
4-[(3-methoxy-benzylidene) amino]-benzoic acid methyl ester

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene for 12h; Reflux; | 100% |
| In N,N-dimethyl-formamide at 100 - 150℃; |
-
-
65854-91-3
N-(4-chlorophenyl)-2,2-dimethylpropionamide

-
-
591-31-1
3-methoxy-benzaldehyde

-
-
1292850-39-5
N-{4-chloro-2-[hydroxy(3-methoxyphenyl)methyl]-phenyl}-2,2-dimethylpropanamide

| Conditions | Yield |
|---|---|
| Stage #1: N-(4-chlorophenyl)-2,2-dimethylpropionamide With sec.-butyllithium In tetrahydrofuran; hexane at -78 - 0℃; for 2h; Stage #2: 3-methoxy-benzaldehyde In tetrahydrofuran; hexane at 0℃; for 0.5h; | 100% |
-
-
591-31-1
3-methoxy-benzaldehyde

-
-
108087-83-8
[(3-methoxyphenyl)methylidene]hydrazine

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In ethanol at 20℃; Cooling; | 100% |
| With hydrazine hydrate In methanol at 20℃; for 1h; | 99% |
| With hydrazine hydrate In methanol at 20℃; for 1h; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-methoxy-benzaldehyde; methylamine In methanol; water for 1h; Stage #2: trimethylsilyl cyanide In methanol; water at 20℃; for 24h; | 100% |

-
-
591-31-1
3-methoxy-benzaldehyde

-
-
94-09-7
p-aminoethylbenzoate

-
-
349456-93-5
4-[(3-methoxy-benzylidene) amino]-benzoic acid methyl ester

| Conditions | Yield |
|---|---|
| toluene-4-sulfonic acid In toluene for 12h; Reflux; | 100% |
m-Anisaldehyde Consensus Reports
m-Anisaldehyde Specification
The m-Anisaldehyde, with the CAS registry number 591-31-1, is also known as Metamethoxybenzaldehyde. It belongs to the product category of Aromatic Aldehydes & Derivatives (substituted). Its EINECS number is 209-712-8. This chemical's molecular formula is C8H8O2 and molecular weight is 136.15. What's more, its systematic name is 3-Methoxybenzaldehyde. Its classification code is Mutation data. This chemical should be sealed and stored in a cool and dry place. It is used as spices, pharmaceutical intermediates and organic intermediates.
Physical properties of m-Anisaldehyde are: (1)ACD/LogP: 1.646; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.65; (4)ACD/LogD (pH 7.4): 1.65; (5)ACD/BCF (pH 5.5): 10.49; (6)ACD/BCF (pH 7.4): 10.49; (7)ACD/KOC (pH 5.5): 187.15; (8)ACD/KOC (pH 7.4): 187.15; (9)#H bond acceptors: 2; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 2 ; (12)Polar Surface Area: 26.3 Å2; (13)Index of Refraction: 1.547; (14)Molar Refractivity: 39.684 cm3; (15)Molar Volume: 125.102 cm3; (16)Polarizability: 15.732×10-24cm3; (17)Surface Tension: 37.22 dyne/cm; (18)Density: 1.088 g/cm3; (19)Flash Point: 100.219 °C; (20)Enthalpy of Vaporization: 46.741 kJ/mol; (21)Boiling Point: 230.761 °C at 760 mmHg; (22)Vapour Pressure: 0.06 mmHg at 25°C.
Preparation of m-Anisaldehyde: this chemical can be prepared by 3-methoxy-benzyl alcohol at the temperature of 28 - 30 °C. This reaction will need reagent cetyltrimethylammonium permanganate and solvent CH2Cl2 with the reaction time of 1 hour. The yield is about 96%.

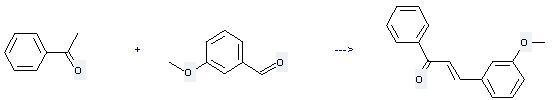
Uses of m-Anisaldehyde: it can be used to produce 3-(3-methoxy-phenyl)-1-phenyl-propenone at the temperature of 35 - 40 °C. It will need reagent aq. NaOH and solvent ethanol with the reaction time of 2.5 hours. The yield is about 81%.
When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes, respiratory system and skin. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need wear suitable protective clothing, gloves and eye/face protection.
You can still convert the following datas into molecular structure:
(1)SMILES: COc1cc(ccc1)C=O
(2)Std. InChI: InChI=1S/C8H8O2/c1-10-8-4-2-3-7(5-8)6-9/h2-6H,1H3
(3)Std. InChIKey: WMPDAIZRQDCGFH-UHFFFAOYSA-N
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View