 This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
Synthetic route

| Conditions | Yield |
|---|---|
| With air; copper(II) oxide; copper(II) carbonate; nickel(II) carbonate In neat (no solvent) storage of thin layer of mixt. of lime nitrogen, soda lime and CuO on air for few days; addn. of catalyst, heating in dry stream of air at 400°C, until oxidn. of C is complete; heating up to 3 h at 450°C; acceleration by min. of H2O vapor;; | 38% |
-
-
10102-43-9
nitrogen(II) oxide

-
B
-
7727-37-9
nitrogen

-
C
-
13477-34-4
calcium(II) nitrate

-
D
-
10024-97-2
dinitrogen monoxide

| Conditions | Yield |
|---|---|
| With calcium oxide 400°C, 21 h; | A 0.9% B n/a C <1 D n/a |
| With CaO 400°C, 21 h; | A 0.9% B n/a C <1 D n/a |


| Conditions | Yield |
|---|---|
| In water addn. of HNO3 to dolomite also dissolves Mg; removal as Mg(OH)2 with CaO;; |


| Conditions | Yield |
|---|---|
| In water dolomite slurry filtered and treated with HNO3; CaCO3 dissolves, and MgCO3 remains undissolved;; | |
| With calcium(II) nitrate In water dolomite dissolved in Ca(NO3)3 soln. containing at most 1% HNO3;; | |
| In water amt. of applied acid insufficient to dissolve also MgCO3;; |

| Conditions | Yield |
|---|---|
| In water addn. of HNO3 to dolomite also dissolves Mg; removal as Mg(OH)2 with Ca(OH)2;; |


| Conditions | Yield |
|---|---|
| In ammonia CaCO3 portion of dolomite dissolves in liquid ammonia containing an equivalent amt. of NH4NO3;; |

| Conditions | Yield |
|---|---|
| In water intermediately formed nitrogen oxides react to form the nitrates;; | |
| In water intermediately formed nitrogen oxides react to form the nitrates;; |

| Conditions | Yield |
|---|---|
| In water |


| Conditions | Yield |
|---|---|
| trituration at moderate temp.;; | |
| trituration at moderate temp.;; |

| Conditions | Yield |
|---|---|
| With nitrogen oxides; Fe(OH)3 or Al(OH)3 or Cr(OH)3 In water absorption of nitrogen oxides in suspensions of heavy metal hydroxides and with CaO; then interaction of the intermediately formed nitrates;; | |
| With nitrogen oxides In water absorption of nitrogen oxides in suspensions of heavy metal hydroxides and with CaO; then interaction of the intermediately formed nitrates;; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) High Pressure; react. of atmospheric N2 and O2 with CaO at 800 - 900°C under high pressure;; | |
| In neat (no solvent) High Pressure; react. of atmospheric N2 and O2 with CaO at 800 - 900°C under high pressure;; |


| Conditions | Yield |
|---|---|
| With phosphate minerals In water Decompn. of phosphate minerals by HNO3; to avoid pptn. of Ca phosphates during neutralization of Ca(NO3)2 solns. because of residual phosphate, neutralization is done with concd. solns. of Ca(NO3)2 satd. with CaO;; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) solving of CaO in molten NH4NO3;; | |
| In neat (no solvent) solving of CaO in molten NH4NO3;; |


| Conditions | Yield |
|---|---|
| byproducts: NH3; H2O; |

| Conditions | Yield |
|---|---|
| In water indirect reaction with HNO3; from destillation gases NH4NO3 is formed by HNO3; NH4NO3 reacted with CaO and NH3 again oxidized to form HNO3 which retuens into the cycle;; |


| Conditions | Yield |
|---|---|
| heating a mixture of AgNO3 and CaO (50 mol %) at 190°C;; |


| Conditions | Yield |
|---|---|
| In neat (no solvent) ratio nitrate : nitrite depending on temp.;; | |
| In neat (no solvent) ratio nitrate : nitrite depending on temp.;; |


| Conditions | Yield |
|---|---|
| In neat (no solvent) use of slake lime dehydrated at 400 - 650°C;; | |
| In neat (no solvent) CaO used in coarse-grained or pelltized form;; | |
| In neat (no solvent) use of quicklime calcined at 700 - 750°C;; |

-
-
80937-33-3
oxygen

-
A
-
7727-37-9
nitrogen

-
B
-
13477-34-4
calcium(II) nitrate

-
C
-
10102-43-9
nitrogen(II) oxide

-
D
-
10102-44-0
Nitrogen dioxide

| Conditions | Yield |
|---|---|
| With nitrogen oxides In neat (no solvent) nitrogen oxides (formed by combustion of air or oxidn. of NH3) react at 230-480 °C with CaO and O2 to form Ca(NO3)2 and Ca(NO2)2, the latter decomposes forming CaO, NO, N2; at >480°C also NO2 forms and oxidizes Ca(NO2)2 to Ca(NO3)2;; | |
| With nitrogen oxides In neat (no solvent) nitrogen oxides (formed by combustion of air or oxidn. of NH3) react at 230-480 °C with CaO and O2 to form Ca(NO3)2 and Ca(NO2)2, the latter decomposes forming CaO, NO, N2; at >480°C also NO2 forms and oxidizes Ca(NO2)2 to Ca(NO3)2;; |


| Conditions | Yield |
|---|---|
| In neat (no solvent) surface oxidation of NH3 at 360 °C on lime surfaces containing 0.2-2.5% NiO or CuO as activators;; | |
| In neat (no solvent) surface oxidation of NH3 at 360 °C on lime surfaces containing 0.2-2.5% NiO or CuO as activators;; |

| Conditions | Yield |
|---|---|
| reaction of NO2 with moist Ca phosphate;; | |
| reaction of NO2 with moist Ca phosphate;; |


-
-
13477-34-4
calcium(II) nitrate

| Conditions | Yield |
|---|---|
| With phosphoric acid byproducts: Na-phosphates; by product of Na phosphate synthesis : treatment of crude phosphate with nitrates (except alkali nitrates and group II nitrates) at elevated temp.;; | |
| With phosphoric acid byproducts: Na-phosphates; by product of Na phosphate synthesis : treatment of crude phosphate with nitrates (except alkali nitrates and group II nitrates) at elevated temp.;; |

| Conditions | Yield |
|---|---|
| With phosphate minerals Decompn. of phosphate minerals by HNO3;; |

-
-
7697-37-2
nitric acid

-
-
13477-34-4
calcium(II) nitrate

| Conditions | Yield |
|---|---|
| With calcium chloride no reaction at low temp.; | 0% |
| With calcium chloride evapn. with excess of HNO3 soln.; | >99 |
| With CaCl2 no reaction at low temp.; | 0% |
| With CaCl2 evapn. with excess of HNO3 soln.; | >99 |

| Conditions | Yield |
|---|---|
| In water react. at bp. of the soln., eventually under pressure;; | |
| With MnO2 or H2SO4 In water with fuming (brown) HNO3 at 20°C or with highly concd.HNO3 in presence of MnO2 or H2SO4 at 50 - 60°C;; | |
| In water react. at bp. of the soln., eventually under pressure;; |

| Conditions | Yield |
|---|---|
| In water Ca(NO3)2 as by-product;; |

| Conditions | Yield |
|---|---|
| With calcium oxide In water addn. of excess of HNO3 to phosphates formed 3Ca(NO3)2*12H2O*HNO3; neutralisation with milk of lime;; pptn.;; | |
| With calcium oxide In water addn. of excess of HNO3 to phosphates formed 3Ca(NO3)2*12H2O*HNO3; neutralisation with milk of lime;; pptn.;; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) | |
| In neat (no solvent) |


-
-
13477-34-4
calcium(II) nitrate

-
-
638-38-0, 993-02-2, 2180-18-9, 15411-95-7, 22981-23-3, 27004-39-3
manganese(II) acetate

| Conditions | Yield |
|---|---|
| With air; glycine In water mixt. blended and heated; calcined at 800-850°C for 2 h; ball milled in ethanol for 24 h; pressed; burn out of binder in vac. at 450°C; sintered at 1100-1550°C for 2 h in air; cooled; XRD; SEM; TEM; | 100% |
-
-
13477-34-4
calcium(II) nitrate

-
-
638-38-0, 993-02-2, 2180-18-9, 15411-95-7, 22981-23-3, 27004-39-3
manganese(II) acetate

| Conditions | Yield |
|---|---|
| With air; glycine In water mixt. blended and heated; calcined at 800-850°C for 2 h; ball milled in ethanol for 24 h; pressed; burn out of binder in vac. at 450°C; sintered at 1100-1550°C for 2 h in air; cooled; XRD; SEM; TEM; | 100% |


| Conditions | Yield |
|---|---|
| With magnesium(II) nitrate; nitric acid; urea mixing stoich. components solns., heating at 85°C for 192 h or at90°C for 72 h or at 95°C for 48 h; XRD, SEM; | 99% |
| With nitric acid; urea mixing stoich. components solns., heating at 85°C for 192 h or at90°C for 72 h or at 95°C for 48 h; XRD, SEM; | 99% |
-
-
67-56-1
methanol

-
-
13477-34-4
calcium(II) nitrate

| Conditions | Yield |
|---|---|
| In methanol; water stirring suspn. of copper compd. in 0.05 M soln. of calcium nitrate in 4:1 mixt. of methanol and water for 1 wk at room temp.; elem. anal.; | 99% |

-
-
366-18-7
[2,2]bipyridinyl

-
-
13477-34-4
calcium(II) nitrate

| Conditions | Yield |
|---|---|
| In water at 60 - 65℃; for 1h; | 97% |


| Conditions | Yield |
|---|---|
| In melt byproducts: NaNO3; melt of NaNO3, 450.+-.5°C, periodically mixed, 40 min; poured into a dish, left to cool, washed repeatedly (10-20 times) with water, dried at 200°C to constant mass, elem. anal.; | 96.34% |
| In water contains Cu or Co when precipitated at pH 8 to 8.5;; | |
| With aq. HNO3; aq. NaOH In water High Pressure; adjusted (aq. HNO3/aq. NaOH) pH of Ca compd. soln. to 1.5, soln. of Mo compd. slowly dropped under vigorous stirring, stirred for 45 min, transferred into autoclave, sealed, heated at 150°C for 6 h, cooled naturally to room temp.; product collected by centrifugation, washed several times with distd. water and abs. ethanol, dried at 80°C for 6 h; | |
| In water contains Cu or Co when precipitated at pH 8 to 8.5;; | |
| In ethylene glycol High Pressure; microwave radiation method; each 0.005 mol of Ca(NO3)2 and Na2MoO4 sep. dissolved in ethylene glycol, two solns. mixed and stirred for 30 min, mixt. trensferred into autoclave, heated at 50% of 600 W microwave for 20min (60 s on and 60 s off); white ppt. filtered, washed (distd. H2O, abs. EtOH), dried (80 °C, 24 h, air); XRD; |

| Conditions | Yield |
|---|---|
| at 95℃; for 6h; | 96% |

| Conditions | Yield |
|---|---|
| In melt byproducts: KNO3; melt of KNO3, 450.+-.5°C, periodically mixed, 40 min; poured into a dish, left to cool, washed repeatedly (10-20 times) with water, dried at 200°C to constant mass, elem. anal.; | 95.69% |

| Conditions | Yield |
|---|---|
| With sodium nitrate In melt mixt. of NaNO3, Ca(NO3)2 and Na2WO4 heated in fused quartz tube at 400°C for 40-50 min with periodic stirring of the melt; poured into porcelain cup; cooled; washed with H2O; dried to const. weight at 200°C; elem. anal.; | 95.24% |
| In water heating at 1000°C, fluorescent product; | |
| In not given |

| Conditions | Yield |
|---|---|
| In solid Kinetics; in electric furnace at 1670 K; XRD; | 95% |
| In solid Kinetics; in electric furnace at 1570 K; | 90% |
| In solid Kinetics; in electric furnace at 1770 K; | 83% |

-
-
13477-34-4
calcium(II) nitrate

| Conditions | Yield |
|---|---|
| In acetonitrile Zn complex dissolved on heating in MeCN, Ca nitrate added in MeCN with stirring, crystn. 2 d; ppt. filtered off, washed with ice-cooled MeCN, dried in vac. at 60 °C, elem. anal.; | 92% |

| Conditions | Yield |
|---|---|
| In ethanol; water aq. soln. Ca(NO3)2 and soln. phen in EtOH were added to aq. soln. Nb complex and KCN; ppt. was collected by centrifugation, washed with H2O, dried under vac.; | A 5% B 90% |


-
-
2359-09-3
5-t-butylisophthalic acid

-
-
13477-34-4
calcium(II) nitrate

| Conditions | Yield |
|---|---|
| In methanol; N,N-dimethyl-formamide High Pressure; mixt. of Cd salt (1 equiv.), acid (2 equiv.) and Ca salt (2 equiv.) in DMF/MeOH (1/1) was heated to 160°C for 4 h in autoclave; heated at160°C for 2 d; cooled to room temp. over 2 d; filtered; washed (DMF, MeOH); dried in air at room temp.; elem. anal.; | 87% |


| Conditions | Yield |
|---|---|
| In methanol; N,N-dimethyl-formamide High Pressure; mixt. of Cd salt (1 equiv.), acid (2 equiv.) and Ca salt (2 equiv.) in DMF/MeOH (1/1) was heated to 160°C for 4 h in autoclave; heated at160°C for 2 d; cooled to room temp. over 2 d; filtered; washed (DMF, MeOH); dried in air at room temp.; elem. anal.; | 86% |


-
-
618-83-7
5-Hydroxyisophthalic acid

-
-
13477-34-4
calcium(II) nitrate

| Conditions | Yield |
|---|---|
| In methanol; N,N-dimethyl-formamide High Pressure; mixt. of Cd salt (1 equiv.), acid (2 equiv.) and Ca salt (2 equiv.) in DMF/MeOH (1/1) was heated to 160°C for 4 h in autoclave; heated at160°C for 2 d; cooled to room temp. over 2 d; filtered; washed (DMF, MeOH); dried in air at room temp.; elem. anal.; | 85% |


-
-
13477-34-4
calcium(II) nitrate

| Conditions | Yield |
|---|---|
| In water soln. of metal salt added to Li salt by stirring; filtered, soln. pptd. with KCl, filtered, washed with EtOH and Et2O, air-dried, elem. anal.; | 80% |

| Conditions | Yield |
|---|---|
| In melt byproducts: CaSO4; ratio of BaSO4 to Ca(NO3)2 = 1:2 because of reversible reaction; quick cooling; crystn. (water); | 75% |
| In melt byproducts: CaSO4; ratio of BaSO4 to Ca(NO3)2 = 1:2 because of reversible reaction; quick cooling; crystn. (water); | 75% |

| Conditions | Yield |
|---|---|
| In water preparation on cation exchange resins, 52% Ca-nitrate soln., 26% NaCl-soln.;; crystn.;; | 70% |
| In water preparation on cation exchange resins, 52% Ca-nitrate soln., 26% NaCl-soln.;; crystn.;; | 70% |
| In water equilibrium in aq. soln.;; |

-
-
13477-34-4
calcium(II) nitrate

| Conditions | Yield |
|---|---|
| Stage #1: 2,2′-{1,2-phenylenebis[oxyacetyl(N,N-diethylthiourea)]}; calcium(II) nitrate; silver nitrate In methanol; water at 20℃; for 0.5h; Stage #2: With triethylamine In methanol; water | 67% |


-
-
13477-34-4
calcium(II) nitrate

-
-
954111-03-6
[Cu(N,N'-2,2-dimethylpropylenedi(3-methoxysalicylideneimine))]2Ca(NO3)2*H2O

| Conditions | Yield |
|---|---|
| In methanol Cu-compd. and 1 equiv. of alkaline earth-compd. were stirred in MeOH for3 h; ppt. was filtered off, dried, elem. anal.; | 65% |
-
-
157143-69-6, 41754-70-5
monoaqua 3-methoxysalicylaldehyde-ethyldiamine copper(II)

-
-
13477-34-4
calcium(II) nitrate

| Conditions | Yield |
|---|---|
| In methanol Cu-compd. and 1 equiv. of alkaline earth-compd. were stirred in MeOH for3 h; ppt. was filtered off, dried, elem. anal.; | 65% |

-
-
13477-34-4
calcium(II) nitrate

-
-
6018-89-9
nickel(II) acetate tetrahydrate

-
-
6147-53-1
cobalt(II) diacetate tetrahydrate

| Conditions | Yield |
|---|---|
| Stage #1: C36H44Au2N2O4P2S2*8H2O; nickel(II) acetate tetrahydrate; cobalt(II) diacetate tetrahydrate With air In methanol at 20℃; for 3h; Stage #2: calcium(II) nitrate In methanol; water at 20℃; for 168h; | A 65% B 16% |


-
-
13477-34-4
calcium(II) nitrate

-
-
7757-83-7
sodium sulfite

-
B
-
7757-82-6
sodium sulfate

-
C
-
7632-00-0
sodium nitrite

| Conditions | Yield |
|---|---|
| In neat (no solvent) formation at heating under glowing;; | A n/a B n/a C 60% |


-
-
13477-34-4
calcium(II) nitrate

-
-
5949-29-1
citric acid monohydrate

| Conditions | Yield |
|---|---|
| In water High Pressure; under aerobic conditions; Ca salt (1.95 mmol), citric acid hydrate (2 mmol), and MnCO3 (1 mmol) dissolved in H2O, mixt. heated 160°C in sealed teflon tube for 3 d; elem. anal.; | 60% |

-
-
7396-77-2
N,N'-bis(3-methoxysalicylidene)ethylenediamine

-
-
13477-34-4
calcium(II) nitrate

-
-
6156-78-1
manganese (II) acetate tetrahydrate

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 2h; | A 60% B 20% |


-
-
13477-34-4
calcium(II) nitrate

| Conditions | Yield |
|---|---|
| With water at 10 - 140℃; for 72h; Temperature; Autoclave; | 60% |
-
-
39929-21-0
(tetrahydrothiophene)gold(I) chloride

-
-
13477-34-4
calcium(II) nitrate

| Conditions | Yield |
|---|---|
| Stage #1: (tetrahydrothiophene)gold(I) chloride; 2,2′-{1,2-phenylenebis[oxyacetyl(N,N-diethylthiourea)]}; calcium(II) nitrate With water In methanol at 20℃; for 0.5h; Stage #2: With triethylamine In methanol at 50℃; for 1h; | 60% |


| Conditions | Yield |
|---|---|
| In acetic anhydride H2O free;; | 59% |
| In acetic anhydride H2O free;; | 59% |
-
-
13477-34-4
calcium(II) nitrate

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

| Conditions | Yield |
|---|---|
| at 95℃; for 24h; High pressure; | 58.7% |


| Conditions | Yield |
|---|---|
| With sodium hydroxide at 100℃; for 72h; pH=7; | 57% |

Related products
Raw Materials
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View

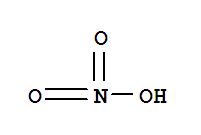








 O,
O, Xi
Xi