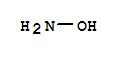Dayang Chem (Hangzhou) Co.,Ltd.
Dayangchem's R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. DayangChem can provide different quantities
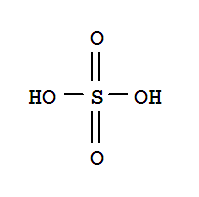
Cas:7803-63-6
Min.Order:1 Kilogram
FOB Price: $3.0
Type:Lab/Research institutions
inquirySimagchem Corporation
Welcome to Simagchem, your partner in China as a premier supply of bulk specialty chemicals for industry and life science. We introduce experienced quality product and exceptional JIT service with instant market intelligence in China to benefit our
Cas:7803-63-6
Min.Order:1 Kilogram
Negotiable
Type:Manufacturers
inquiryChemwill Asia Co., Ltd.
Our main production base is located in Xuzhou industry park. We are certified both to the ISO 9001 and ISO 14001 Standards, have a safety management system in place.Our R&D team masters core technology for process-design of target building block
Cas:7803-63-6
Min.Order:5 Kiloliter
FOB Price: $1.2 / 5.0
Type:Manufacturers
inquiryHangzhou Ocean Chemical Co., Ltd.
Ocean inorganic department is a professional supplier and exporter engaging in inorganic chemical materials and metal organic compounds. Over the past years, our company is committed to improving the product quality and developing new products, in
Cas:7803-63-6
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHangzhou J&H Chemical Co., Ltd.
J&H CHEM R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. J&H CHEM has some Manufacturing base in Jia
Cas:7803-63-6
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryZibo Hangyu Biotechnology Development Co., Ltd
Zibo Hangyu Biotechnology Development Co., Ltd is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemi
Cas:7803-63-6
Min.Order:10 Gram
FOB Price: $100.0
Type:Lab/Research institutions
inquiryKono Chem Co.,Ltd
high purity lowest priceAppearance:solid or liquid Storage:in sealed air resistant place Package:drum and bag Application:for pharma use Transportation:by sea or air Port:Beijing or Guangzhou
Henan Allgreen Chemical Co.,Ltd
high quality Storage:Sealed, dry, microtherm , avoid light and smell. Package:According to the demand of customer Application:Organic synthesis Transportation:by air or by sea
Aecochem Corp.
Our clients, like BASF,CHEMO,Brenntag,ASR,Evonik,Merck and etc.Appearance:COA Storage:in stock Application:MSDS/TDS
Henan Tianfu Chemical Co., Ltd.
1.Our services:A.Supply sampleB.The packing also can be according the customers` requirmentC.Any inquiries will be replied within 24 hoursD.we provide Commerical Invoice, Packing List, Bill of loading, COA , Health certificate and Origin certificate.
Cas:7803-63-6
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryChongqing Changfeng Chemical Co., Ltd.
High purity ammonium bisulfate with stable supply Application:used as general reagent Port:Shanghai port
Zhuozhou Wenxi import and Export Co., Ltd
Product Description Description & Specification Category Pharmaceutical Raw Materials, Fine Chemicals, Bulk drug Standard Medical standard
Cas:7803-63-6
Min.Order:1 Kilogram
FOB Price: $112.0
Type:Trading Company
inquiryAntimex Chemical Limied
Ansciep Chemical is a professional enterprise manufacturing and distributing fine chemicals and speciality chemicals. We have been dedicated to heterocycle compounds and phenyl rings for tens of years. This is our mature product for export. Our quali
Cas:7803-63-6
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryHunan Russell Chemicals Technology Co.,Ltd
low price and high purityAppearance:solid or liquid Storage:in sealed air resistant place Package:As customer require Application:Pharma;Industry;Agricultural Transportation:by sea or by airplane Port:any port in China
Cas:7803-63-6
Min.Order:0
Negotiable
Type:Trading Company
inquiryBeantown Chemical
in stock Application:7803-63-6
Cas:7803-63-6
Min.Order:0
Negotiable
Type:Trading Company
inquiryHENAN SUNLAKE ENTERPRISE CORPORATION
Ammonium hydrogen sulfate Basic information Product Name: Ammonium hydrogen sulfate Synonyms: Sulfuricacid,monoammoniumsalt;AMMONIUM BISULFAT
Hebei Ruishun Trade Co.,Ltd
Profile HeBei Ruishun Trade Co.LTD, registered capital one million,have a production of pharmaceutical raw materials, pharmaceutical raw materials factory reagent r&d center,Seek development by credit
Cas:7803-63-6
Min.Order:500 Gram
FOB Price: $1.0
Type:Trading Company
inquiryXiamen BaiFuchem Co.,Ltd
BaiFuChem is a Professional chemical raw material supplier in China, our main products include Biochemical , Pharma Intermediate and Organic chemical etc. BaiFuChem have wealth of products,experience , expertise and state-of-the-art
Cas:7803-63-6
Min.Order:1 Kilogram
Negotiable
Type:Other
inquiryChemical Co.Ltd
1.Professional manufacturer 2.Best price 3.Fast delivery 4.Prompt before and after sale service 5.Quality guarantee 6.Reliable and truthworthy supplier Appearance:Standardized Look(Large crystal, Powder, Solid, Liquid) Storage:Room temper
Cas:7803-63-6
Min.Order:1 Kilogram
Negotiable
Type:Trading Company
inquiryHangzhou Share Chemical Co., Ltd
At Share Chemical Company, we scrupulously abide by our policy of “Excellent Quality at a Reasonable Price”. We strive to satisfy all of our customers by providing the finest quality products supported by the finest in customer servi
Cas:7803-63-6
Min.Order:1 Gram
Negotiable
Type:Other
inquiryORCHID CHEMICAL SUPPLIES LTD
Ammonium bisulfate CAS#7803-63-6 Storage:Keep container tightly closed in a dry, cool and well-ventilated place. Package:25Kg/drum, 150Kg/drum, 200kg/drum, According to request. Transportation:By sea, by air.
Shanghai united Scientific Co.,Ltd.
United Scientific Company Located in Shanghai of China , is a competitive player in the global specialty and fine chemical market. Fenghua has both the expertise and flexibility to produce a wide range of chemicals. Focusing on developing the innovat
Cas:7803-63-6
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryChungking Joyinchem Co., Ltd.
Joyinchem have been committed to chemical supply for several years and have built good cooperation records with multinational chemical corporations and importers from all over the world. Our services include:-Spot goods-Contract manufacturing-Custom
Cas:7803-63-6
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryNanjing Chemlin Chemical Co., Ltd.
please contact us to confirm the required quantity and quote, we will provide you with COA,NMR,HPLC and other relevant informationAppearance:White crystals Storage:Store in a cool place. Keep container tightly closed in a dry and well-ventilated plac
Nanjing Raymon Biotech Co., Ltd.
Sulfuric acid, ammoniumsalt (1:1) Storage:keep in dry and cool condition Package:25kg or according to cutomer's demand Application:Chemical research/pharma intermediate Transportation:By Sea,by Air,By courier like DHL or Fedx. Port:Shanghai/Shenzhen
Hangzhou Yierdechem Co. Ltd
With about ten years experiences in the field of pharmaceutical chemicals, Yierdechem has established solid business cooperation relationships with many large companys worldwide.As a leading supplier of API and pharmaceutical intermediates, holds its
Debye Scientific
Debyesci is here who supplied several kinds of chemical products to global pharmaceutical, drug discovery, agrochemical and biotechnology industries for four yearsOur key scientific leadership team has gained experience in top research and developmen
Cas:7803-63-6
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryNovaChemistry
high purity Application:Drug intermediates Materials intermediates and active molecules
Synthetic route

| Conditions | Yield |
|---|---|
| With air In solid by standing in air for 24 h; | 99% |
-
-
13954-94-4
trisulfimide

-
A
-
7803-63-6
ammonium bisulfate

-
B
-
7664-93-9
sulfuric acid

-
C
-
7803-58-9
SULFAMIDE

| Conditions | Yield |
|---|---|
| With water heat of evapn., 2 h, 2 M HCl; | A n/a B 67% C n/a |
| With H2O heat of evapn., 2 h, 2 M HCl; | A n/a B 67% C n/a |


| Conditions | Yield |
|---|---|
| In sulfuric acid heating with an excess of H2SO4;; |


| Conditions | Yield |
|---|---|
| With ammonia; water; oxygen; vanadia In further solvent(s) 150-200 °C, in molten equimolar mixture of NH4HSO4 and NaHSO4 dissolving V2O5; transition metal sulfates enhances activity; |

| Conditions | Yield |
|---|---|
| With water; chlorine byproducts: NH4Cl, HCl; with NH3 excess; |

| Conditions | Yield |
|---|---|
| heating at 180°C; |

-
-
1333-74-0
hydrogen

-
-
10102-43-9
nitrogen(II) oxide

-
A
-
227934-46-5
nitrous oxide

-
B
-
7803-63-6
ammonium bisulfate

-
C
-
10046-00-1
hydroxylamine sulfate

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal In sulfuric acid Kinetics; 40 °C, P(H2) = 50 kPa, P(NO) = 10 kPa; |


-
-
7803-63-6
ammonium bisulfate

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water satd. soln. of neutral sulfate is distd. with aq. HCl;; | |
| With sulfuric acid In sulfuric acid cooling a warm solution of (NH4)2SO4 in H2SO4; | |
| Pt boat; 300 to 320 ° C and for a short period at red heat; |

| Conditions | Yield |
|---|---|
| 200 to 250°C; | |
| heating in open vessel; ambient or reduced pressure; | |
| In neat (no solvent) Kinetics; Thermal decomposition.; | |
| 200 to 250°C; | |
| In neat (no solvent) decompn. of (NH4)2SO4 into NH3 and NH4HSO4 at 300-455°C was studied; TG and DSC; |

-
B
-
7803-63-6
ammonium bisulfate

| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: NH3, H2O; 150°C; IR spectroscopy, X-ray diffraction; |


-
A
-
7803-63-6
ammonium bisulfate

| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: NH3, H2O; 250°C; IR spectroscopy, X-ray diffraction; |


| Conditions | Yield |
|---|---|
| In water byproducts: H2O, CuSO4; |

-
-
7664-93-9
sulfuric acid

-
-
57-13-6
urea

-
A
-
7803-63-6
ammonium bisulfate

-
B
-
5329-14-6
aminosulfonic acid

-
C
-
7783-05-3
disulfuric acid

| Conditions | Yield |
|---|---|
| In sulfuric acid byproducts: CO2; heating with excess H2SO4 (100%/130-140°C); evolution of CO2;; |

-
-
82324-00-3
hydroxyl ammonium amidosulfonate

-
A
-
7803-63-6
ammonium bisulfate

-
B
-
7727-37-9
nitrogen

-
C
-
10102-43-9
nitrogen(II) oxide

-
D
-
10024-97-2
dinitrogen monoxide

| Conditions | Yield |
|---|---|
| in a larger amt. at 95°C, in a smaller amt. at 110°C; | |
| in a larger amt. at 95°C, in a smaller amt. at 110°C; |


| Conditions | Yield |
|---|---|
| In not given byproducts: HCl; | |
| In not given byproducts: HCl; |
-
-
7446-09-5
sulfur dioxide

-
-
7803-49-8
hydroxylamine

-
A
-
7803-63-6
ammonium bisulfate

-
B
-
25278-06-2, 5329-14-6
sulfamic acid

| Conditions | Yield |
|---|---|
| In water Kinetics; at pH=5; followed by polarography; |


| Conditions | Yield |
|---|---|
| With H2O In water byproducts: H2O2; hydrolysis on boiling;; |
-
-
13598-45-3
(hydroxylamino)sulfonic acid

-
A
-
7803-63-6
ammonium bisulfate

-
B
-
7722-84-1
dihydrogen peroxide

-
C
-
80937-33-3
oxygen

| Conditions | Yield |
|---|---|
| With water In water hydrolysis on boiling;; | A 0% B 0% C 0% |
| With H2O In water hydrolysis on boiling;; | A 0% B 0% C 0% |


| Conditions | Yield |
|---|---|
| With H2O In water decompn.;; |

| Conditions | Yield |
|---|---|
| by hydrolysis; purification from NH4HSO4 by precipitation with baryta water,then evapn.over H2SO4 under reduced pressure; | |
| by hydrolysis; purification from NH4HSO4 by precipitation with baryta water,then evapn.over H2SO4 under reduced pressure; |

| Conditions | Yield |
|---|---|
| In melt passing dry ammonia through molten (NO)2S2O7;; | |
| In melt passing dry ammonia through molten (NO)2S2O7;; |


| Conditions | Yield |
|---|---|
| In neat (no solvent) passing dry ammonia(NO)2S2O7 (liquidation);; |


-
-
7803-63-6
ammonium bisulfate

| Conditions | Yield |
|---|---|
| deliquescent by air;ammonium hydrosulfate crystallizes from the liquid so formed; | |
| deliquescent by air;ammonium hydrosulfate crystallizes from the liquid so formed; |

| Conditions | Yield |
|---|---|
| In water by slow evapn. of aq. solns. of (NH4)2SO4:H2SO4 stoich. mixt. at room temp.; detd. by XRD; | |
| In water slow evapn. from aq. soln.; |

| Conditions | Yield |
|---|---|
| With air In neat (no solvent) vigorous hydrolysis on heating to 130-140°C in common (moist) air;; | |
| With water In water hydrolysis described; degree of hydrolysis depending on temp. and time;; | |
| With water In water hydrolysis on heating for longer period;; |

-
-
7803-63-6
ammonium bisulfate

| Conditions | Yield |
|---|---|
| heating by 10 Hgmm; at 300 °C melting and foaming; no more changing until 300 °C.; | |
| With water In water byproducts: O2; slow by room temp.,fast at elevated temp.; | |
| With water In water Kinetics; byproducts: O2; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) decompn. by heating (1h/400°C);; |


| Conditions | Yield |
|---|---|
| With air In melt two salts was reacted in melt in air; | A n/a B n/a C 92% |


| Conditions | Yield |
|---|---|
| byproducts: H2SO4; after 6 hours; | 30% |
| byproducts: H2SO4; after 6 hours; | 30% |

| Conditions | Yield |
|---|---|
| In water ZnS (pptd. in the heat, hot washed, well dried and pulverized); amount of H2S depends on concn. of acidic soln.; dilution: 1 mol NH4HSO4 in 4 l;; | 2.06% |
| In water ZnS (pptd. in the heat, hot washed, well dried and pulverized); amount of H2S depends on concn. of acidic soln.; dilution: 1 mol NH4HSO4 in 8 l;; | 2.16% |
| In water ZnS (pptd. in the heat, hot washed, well dried and pulverized); amount of H2S depends on concn. of acidic soln.; dilution: 1 mol NH4HSO4 in 1 l;; | 1.7% |
| In water ZnS (pptd. in the heat, hot washed, well dried and pulverized); amount of H2S depends on concn. of acidic soln.; dilution: 1 mol NH4HSO4 in 2 l;; | 1.85% |
-
-
7803-63-6
ammonium bisulfate

-
-
141-43-5
ethanolamine

-
-
926-39-6
sulfuric acid mono-(2-amino-ethyl ester)

| Conditions | Yield |
|---|---|
| With ammonium sulfate |

| Conditions | Yield |
|---|---|
| In neat (no solvent) heating;; | |
| In water distillation of HCl;; | |
| In neat (no solvent) heating at 280°C;; | |
| In neat (no solvent) passing NH4Cl vapor over NH4HSO4;; | |
| In water |


| Conditions | Yield |
|---|---|
| With potassium sulfate In sulfuric acid byproducts: K2S2O8; Electrolysis; Pietzsch-Adolph process: anode: Pt/cathode: carbon (asbestos cord wound); presence of small amount of KHSO4;; | |
| With K2SO4 In sulfuric acid byproducts: K2S2O8; aq. H2SO4; Electrolysis; Pietzsch-Adolph process: anode: Pt/cathode: carbon (asbestos cord wound); presence of small amount of KHSO4;; |


| Conditions | Yield |
|---|---|
| With fluorine In water reaction by influence of F2 on the aq. soln., higher yields at decreasing influence times of F2;; |

| Conditions | Yield |
|---|---|
| With potassium hydrogensulfate In sulfuric acid byproducts: K2S2O8; Electrolysis; Pietzsch-Adolph process: electrolytically yielded (NH4)2S2O8 is reacted with KHSO4 to form solid K2S2O8;; | |
| In sulfuric acid Electrolysis; Loewenstein Riedel process: hydrolysis to H2O2 on distillation; Pt anode/diaphragm;; | |
| In sulfuric acid Electrolysis; Loewenstein-Riedel process: hydrolysis to H2O2 by distillation;; |

| Conditions | Yield |
|---|---|
| 220°C, 15 to 60 min; |

-
-
7803-63-6
ammonium bisulfate

-
-
7647-14-5
sodium chloride

-
A
-
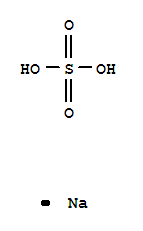
7681-38-1
sodium hydrogen sulfate

-
B
-
7664-41-7
ammonia

| Conditions | Yield |
|---|---|
| In neat (no solvent) addn. of NaCl to molten NH4HSO4 leads to the formation of NH4NaSO4 and HCl, which reacts to NH3 and NaHSO4 by further heating;; | |
| In neat (no solvent) addn. of NaCl to molten NH4HSO4 leads to the formation of NH4NaSO4 and HCl, which reacts to NH3 and NaHSO4 by further heating;; |


| Conditions | Yield |
|---|---|
| 220°C, 15 to 60 min; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) iron shavings are added to molten (NH4)HSO4;; product mixture obtained;; | |
| In neat (no solvent) iron shavings are added to molten (NH4)HSO4;; product mixture obtained;; |


| Conditions | Yield |
|---|---|
| In melt Co(II)-salt is molten in 5 - 6 parts of NH4HSO4;; crystn.;; |
-
-
7789-77-7
calcium hydrogen phosphate

-
-
7803-63-6
ammonium bisulfate

-
-
7722-76-1
ammonium dihydrogen phosphate

| Conditions | Yield |
|---|---|
| CaHPO4 was treated with equivalent amount of NH4HSO4;; | |
| CaHPO4 was treated with equivalent amount of NH4HSO4;; |
-
-
7803-63-6
ammonium bisulfate

| Conditions | Yield |
|---|---|
| In neat (no solvent) FeSO4 is added to molten (NH4)HSO4;; product mixture obtained;; | |
| In neat (no solvent) FeSO4 is added to molten (NH4)HSO4;; product mixture obtained;; |

-
-
7803-63-6
ammonium bisulfate

-
-
7647-14-5
sodium chloride

-
A
-
7647-01-0
hydrogenchloride

-
B
-
7681-38-1
sodium hydrogen sulfate

-
C
-
7664-41-7
ammonia

| Conditions | Yield |
|---|---|
| In not given formation of NaNH4SO4 on heating NH4HSO4 with NaCl at 400 °C; reaction of NaNH4SO4 forming NH3 and NaHSO4;; formation of a ammonia-free product;; | |
| In not given formation of NaNH4SO4 on heating NH4HSO4 with NaCl; reaction of NaNH4SO4 forming NH3 and NaHSO4;; | |
| In not given formation of NaNH4SO4 on heating NH4HSO4 with NaCl at 400 °C; reaction of NaNH4SO4 forming NH3 and NaHSO4;; formation of a ammonia-free product;; | |
| In not given formation of NaNH4SO4 on heating NH4HSO4 with NaCl; reaction of NaNH4SO4 forming NH3 and NaHSO4;; |

Related products
Downstream Products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View