-
Name
7-Ethylindole
- EINECS -0
- CAS No. 22867-74-9
- Article Data8
- CAS DataBase
- Density 1.058
- Solubility INSOLUBLE
- Melting Point 230 ºC
- Formula C10H11N
- Boiling Point 281.9 °C at 760 mmHg
- Molecular Weight 145.204
- Flash Point 119.9 °C
- Transport Information
- Appearance Pale yellow clear liquid
- Safety S23;S24/25
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms TIMTEC-BB SBB007573;7-ETHYLINDOLE;7-ETHYL-INDOL;7-ETHYLINDOLE(ETODOLAC INTERMEDIATE);7-Ethylindole,98+%;7-ETHYLCAMPTOTHECINSM-47-ETHYLINDOLE
- PSA 15.79000
- LogP 2.73030
Synthetic route

| Conditions | Yield |
|---|---|
| With triphenylphosphine; tin(ll) chloride; ruthenium trichloride In 1,4-dioxane; water at 180℃; for 20h; Cyclization; | 87% |
| With tin(ll) chloride; ruthenium trichloride; triphenylphosphine In 1,4-dioxane; water at 180℃; for 20h; | 87% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -40℃; for 1h; Inert atmosphere; | 41% |
-
-
343791-43-5
1-(2-Amino-3-ethyl-phenyl)-2-chloro-ethanone

-
-
22867-74-9
7-ethyl-1H-indole

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In 1,4-dioxane; water for 1h; Heating; Yield given; |

| Conditions | Yield |
|---|---|
| With silica gel; zirconium(IV) oxide at 309.85℃; | 54.2 % Chromat. |
-
-
578-54-1
ortho-ethylaniline

-
-
22867-74-9
7-ethyl-1H-indole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1.) BCl3, AlCl3, 2.) 2 N HCl / 1.) C6H6, CH2Cl2, reflux, 3 h, 2.) C6H6, CH2Cl2, 80 deg C, 45 min 2: NaBH4 / dioxane; H2O / 1 h / Heating View Scheme |

-
-
64-17-5
ethanol

-
-
109-89-7
diethylamine

-
-
108-90-7
chlorobenzene

-
-
98-83-9
isopropenylbenzene

-
-
22867-74-9
7-ethyl-1H-indole

| Conditions | Yield |
|---|---|
| With triethylamine |


| Conditions | Yield |
|---|---|
| With potassium In triethylamine |

-
-
22867-74-9
7-ethyl-1H-indole

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
396074-57-0
7-ethyl-1H-indole-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With dmap In acetonitrile | 100% |
| With dmap In acetonitrile | 100% |
| With dmap In acetonitrile at 20℃; for 3h; | 82% |
-
-
22867-74-9
7-ethyl-1H-indole

-
-
431-46-9
2,2,2-trifluoro-1-methoxy-ethanol

-
-
24313-88-0
3,4,5-Trimethoxyaniline

-
-
1132829-55-0
N-((R)-2,2,2-trifluoro-1-(7-ethyl-1H-indol-3-yl)ethyl)-3,4,5-trimethoxybenzenamine

| Conditions | Yield |
|---|---|
| With (S)-3,3'-bis(2,4,6-tri-iso-propylphenyl)-1,1'-binaphthyl-2,2'-diyl hydrogenphosphate In dichloromethane at 20℃; for 48h; Friedel Crafts aminoalkylation; Molecular sieve; optical yield given as %ee; enantioselective reaction; | 99% |

-
-
22867-74-9
7-ethyl-1H-indole

-
-
434-45-7
1,1,1-trifluoroacetophenone

-
-
1160936-86-6
2,2,2-trifluoro-1-(7-ethyl-1H-indole-3-yl)-1-phenylethanol

| Conditions | Yield |
|---|---|
| With (S)-3,3'-bis(2,4,6-tri-iso-propylphenyl)-1,1'-binaphthyl-2,2'-diyl hydrogenphosphate In dichloromethane at 25℃; for 48h; optical yield given as %ee; enantioselective reaction; | 99% |
-
-
22867-74-9
7-ethyl-1H-indole

-
-
372-31-6
ethyl 4,4,4-trifluoroacetoacetate

| Conditions | Yield |
|---|---|
| With (S)-3,3'-bis(2,4,6-tri-iso-propylphenyl)-1,1'-binaphthyl-2,2'-diyl hydrogenphosphate In dichloromethane at 20℃; for 76h; Friedel-Crafts reaction; optical yield given as %ee; enantioselective reaction; | 99% |
-
-
22867-74-9
7-ethyl-1H-indole

-
-
107969-78-8, 6395-86-4
methyl 4-phenyl-2-oxo-3-butenoate

| Conditions | Yield |
|---|---|
| With C35H48N4O4; samarium(III) trifluoromethanesulfonate In dichloromethane at -20℃; for 10h; asymmetric Friedel-Crafts alkylation; Inert atmosphere; optical yield given as %ee; | 99% |
-
-
22867-74-9
7-ethyl-1H-indole

-
-
13081-18-0
ethyl-3,3,3-trifluoropyruvate

-
-
1262999-77-8
3,3,3-trifluoro-2-hydroxy-2-(7-ethyl-1H-indol-3-yl)-propionic acid ethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 7-ethyl-1H-indole With C33H52N4O4; zinc trifluoromethanesulfonate In dichloromethane at 35℃; for 0.5h; Stage #2: ethyl-3,3,3-trifluoropyruvate In dichloromethane at 0℃; for 0.5h; Friedel Crafts alkylation; optical yield given as %ee; enantioselective reaction; | 99% |
-
-
22867-74-9
7-ethyl-1H-indole

-
-
13081-18-0
ethyl-3,3,3-trifluoropyruvate

-
-
1179934-56-5
3,3,3-trifluoro-2-hydroxy-2-(7-ethyl-1H-indol-3-yl)-propionic acid ethyl ester

| Conditions | Yield |
|---|---|
| In 1,1,1,3,3-pentafluorobutane at 20℃; for 0.5h; Inert atmosphere; | 97% |
| With triethylamine In dichloromethane at 0℃; Friedel Crafts alkylation; |
-
-
22867-74-9
7-ethyl-1H-indole

-
-
13081-18-0
ethyl-3,3,3-trifluoropyruvate

-
-
1262999-82-5
3,3,3-trifluoro-2-hydroxy-2-(7-ethyl-1H-indol-3-yl)-propionic acid ethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 7-ethyl-1H-indole With C33H52N4O4; zinc trifluoromethanesulfonate In dichloromethane at 35℃; for 0.5h; Stage #2: ethyl-3,3,3-trifluoropyruvate In dichloromethane at 0℃; for 0.5h; Friedel Crafts alkylation; optical yield given as %ee; enantioselective reaction; | 96% |
-
-
22867-74-9
7-ethyl-1H-indole

| Conditions | Yield |
|---|---|
| Stage #1: diisopropyl (1,1‐dioxidobenzo[d]isothiazol‐3‐yl)phosphonate With (S)-3,3'-bis[3,5-di(trifluoromethyl)phenyl]-5,5',6,6',7,7',8,8'-octahydro-1,1'-binaphthyl-2,2'-diylphosphoric acid In 1,3,5-trimethyl-benzene at 30℃; for 0.166667h; Friedel-Crafts Alkylation; Schlenk technique; Stage #2: 7-ethyl-1H-indole In 1,3,5-trimethyl-benzene at 30℃; Friedel-Crafts Alkylation; Schlenk technique; enantioselective reaction; | 96% |

| Conditions | Yield |
|---|---|
| In water at 80℃; for 5h; | 96% |


| Conditions | Yield |
|---|---|
| Stage #1: 7-ethyl-1H-indole With C43H64N4O4; scandium tris(trifluoromethanesulfonate); ortho-chlorobenzoic acid In dichloromethane at 30℃; for 1h; Inert atmosphere; Stage #2: glyoxylic acid ethyl ester In dichloromethane at -20℃; for 12h; Friedel Crafts alkylation; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 95% |
-
-
22867-74-9
7-ethyl-1H-indole

-
-
32133-82-7
Martins sulfurane

-
-
1260119-59-2
7-ethyl-3-(diphenylsulfonio)indolide

| Conditions | Yield |
|---|---|
| In toluene at 20℃; for 2h; Inert atmosphere; | 95% |
| In toluene at 25℃; for 2h; Schlenk technique; Inert atmosphere; | 95% |
-
-
22867-74-9
7-ethyl-1H-indole

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

| Conditions | Yield |
|---|---|
| Stage #1: N,N-dimethyl-formamide With trichlorophosphate at 0℃; for 0.166667h; Stage #2: 7-ethyl-1H-indole at 45℃; for 2h; | 95% |
| Stage #1: N,N-dimethyl-formamide With trichlorophosphate for 1.5h; Cooling with ice; Stage #2: 7-ethyl-1H-indole at 20℃; Cooling with ice; Stage #3: With water; potassium hydroxide at 105℃; for 1h; |

| Conditions | Yield |
|---|---|
| With Selectfluor In acetonitrile at 20℃; for 0.2h; | 94% |
| With 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione In acetonitrile at 20℃; for 0.166667h; | 94% |
| With toluene-4-sulfonic acid In methanol at 20℃; for 0.5h; regioselective reaction; | 86% |
-
-
22867-74-9
7-ethyl-1H-indole

-
-
699-18-3, 32782-45-9
2-[(E)-2-nitroethenyl]furan

-
-
1042446-56-9
7-ethyl-3-(1-(furan-2-yl)-2-nitroethyl)-1H-indole

| Conditions | Yield |
|---|---|
| With β‐cyclodextrin In methanol; water at 50℃; for 2.4h; | 94% |
| With tetrabutylammomium bromide In acetonitrile for 0.166667h; Michael addition reaction; Heating; | 93% |
-
-
22867-74-9
7-ethyl-1H-indole

-
-
102-96-5
(2-nitroethenyl)benzene

-
-
1042446-55-8
7-ethyl-3-(2-nitro-1-phenylethyl)-1H-indole

| Conditions | Yield |
|---|---|
| With zinc diacetate In ethanol at 20℃; for 0.366667h; Michael addition; | 94% |
-
-
455-19-6
4-Trifluoromethylbenzaldehyde

-
-
2033-24-1
cycl-isopropylidene malonate

-
-
22867-74-9
7-ethyl-1H-indole

-
-
1202463-59-9
5-{(7-Ethyl-1H-indol-3-yl)[4-(trifluoromethyl)phenyl]methyl}-2,2-dimethyl-1,3-dioxane-4,6-dione

| Conditions | Yield |
|---|---|
| rac-Pro-OH In acetonitrile at 20℃; | 94% |

-
-
22867-74-9
7-ethyl-1H-indole

-
-
119072-55-8, 7188-38-7
tert-butylisonitrile

-
-
1370337-44-2
N-tert-butyl-7-ethyl-1H-indole-3-carboxamide

| Conditions | Yield |
|---|---|
| With water; copper diacetate; palladium diacetate; trifluoroacetic acid In tetrahydrofuran at 70℃; for 1h; Sealed tube; Air atmosphere; | 94% |

| Conditions | Yield |
|---|---|
| With manganese(IV) oxide; iodine; oxygen at 80℃; for 16h; Schlenk technique; | 93% |
| With Selectfluor In acetonitrile at 20℃; for 0.416667h; | 89% |
-
-
5153-67-3
nitrostyrene

-
-
22867-74-9
7-ethyl-1H-indole

-
-
1042446-55-8
7-ethyl-3-(2-nitro-1-phenylethyl)-1H-indole

| Conditions | Yield |
|---|---|
| With β‐cyclodextrin In methanol; water at 50℃; for 2.2h; | 93% |
| With tetrabutylammomium bromide In acetonitrile for 0.166667h; Michael addition reaction; Heating; | 92% |
| With diphenylphosphate In dichloromethane; benzene at -30℃; Friedel-Crafts Alkylation; Molecular sieve; Sealed tube; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| Stage #1: 7-ethyl-1H-indole With potassium hydroxide In N,N-dimethyl-formamide at 21℃; for 1h; Stage #2: 1-bromo-butane In N,N-dimethyl-formamide at 50℃; | 93% |

| Conditions | Yield |
|---|---|
| indium(III) bromide In 1,2-dichloro-ethane for 6h; Heating; | 92% |

| Conditions | Yield |
|---|---|
| With sodium tetrakis[(3,5-di-trifluoromethyl)phenyl]borate In water at 30℃; for 8h; | 92% |
-
-
22867-74-9
7-ethyl-1H-indole

-
-
766-77-8
Dimethylphenylsilane

-
-
1343516-65-3
7-ethyl-1-dimethylphenylsilyl-1H-indole

| Conditions | Yield |
|---|---|
| With pyridine; zinc trifluoromethanesulfonate In propiononitrile at 100℃; for 40h; Inert atmosphere; | 92% |
-
-
22867-74-9
7-ethyl-1H-indole

-
-
36004-04-3
ethyl (E)-1-phenylbut-1-en-3-ol

| Conditions | Yield |
|---|---|
| With indium(III) bromide In 1,2-dichloro-ethane at 20℃; for 0.5h; | 91% |

| Conditions | Yield |
|---|---|
| With bismuth(lll) trifluoromethanesulfonate In acetonitrile at 20℃; for 2.5h; | 91% |
-
-
22867-74-9
7-ethyl-1H-indole

-
-
5670-66-6, 5670-67-7, 3156-39-6
1-nitro-2-(2-nitrovinyl)benzene

-
-
1173110-94-5
C18H17N3O4

| Conditions | Yield |
|---|---|
| With zinc diacetate In ethanol at 20℃; for 0.416667h; Michael addition; | 91% |
-
-
623-73-4
diazoacetic acid ethyl ester

-
-
22867-74-9
7-ethyl-1H-indole

| Conditions | Yield |
|---|---|
| With indium(III) bromide In dichloromethane at 20℃; for 5.5h; | 90% |

| Conditions | Yield |
|---|---|
| With bismuth(lll) trifluoromethanesulfonate In acetonitrile at 20℃; for 0.333333h; | 90% |

| Conditions | Yield |
|---|---|
| copper(II) bis(trifluoromethanesulfonate) In various solvent(s) at 20℃; for 3.5h; | 90% |
7-Ethylindole Chemical Properties
The density of 7-Ethylindole(22867-74-9) is 1.058 g/mL at 20 °C(lit.) and it has a boiling point of 230 °C . The refractive index is about 1.603. Its flash point is 230°C.
The chemical synonyms of 7-Ethylindole(22867-74-9) are TIMTEC-BB SBB007573;7-ETHYLINDOLE;7-ETHYL-INDOL;7-ETHYLINDOLE(ETODOLAC INTERMEDIATE);7-Ethylindole,98+%;7-ETHYLCAMPTOTHECIN
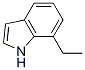
The molecular structure of 7-Ethylindole(22867-74-9):
7-Ethylindole Toxicity Data With Reference
LD50/LC50: RTECS: Not available.
Carcinogenicity: 7-ETHYLINDOLE, 97% - Not listed as a carcinogen by ACGIH, IARC, NTP, or CA Prop 65.
7-Ethylindole Safety Profile
WGK Germany 3
7-Ethylindole Specification
Conditions to Avoid: Incompatible materials.
Incompatibilities with Other Materials Strong oxidizing agents.
Hazardous Decomposition Products Nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization Has not been reported.
Related Products
- 7-Ethylindole
- 7-Ethylindole-3-carboxaldehyde
- 22868-76-4
- 228710-82-5
- 22871-55-2
- 22871-58-5
- 228719-18-4
- 22873-28-5
- 22874-79-9
- 22876-19-3
- 22876-20-6
- 22876-22-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View