-
Name
Benzonitrile
- EINECS 202-855-7
- CAS No. 100-47-0
- Article Data2160
- CAS DataBase
- Density 1.032 g/cm3
- Solubility 10 g/L (100 °C)
- Melting Point -13 °C
- Formula C7H5N
- Boiling Point 191.099 °C at 760 mmHg
- Molecular Weight 103.123
- Flash Point 71.667 °C
- Transport Information UN 2224 6.1/PG 2
- Appearance colourless liquid
- Safety 23
- Risk Codes 21/22
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Benzene,cyano-;Benzenecarbonitrile;Benzenenitrile;Benzoic acid nitrile;Benzonitril;Cyanobenzene;NSC 8039;Phenyl cyanide;
- PSA 23.79000
- LogP 1.55828
Synthetic route

| Conditions | Yield |
|---|---|
| With phosphorus pentoxide In methanol at 20℃; for 1h; | 100% |
| With oxalyl dichloride; triethylamine; Triphenylphosphine oxide In acetonitrile at 20℃; for 0.166667h; Solvent; | 98% |
| With trimethylsilylphosphate for 0.666667h; Heating; | 97% |

| Conditions | Yield |
|---|---|
| With iron(III) chloride; hydroxylamine hydrochloride In dimethyl sulfoxide Molecular sieve; | 100% |
| With [bis(acetoxy)iodo]benzene; ammonium bicarbonate In methanol; water at 36℃; for 12h; Sealed tube; | 100% |
| With N-(4-sulphonic acid)butylpyridinium hydrogen sulphate; 1-sulfobutylpyridine hydrogensulfate hydroxylamine In toluene at 100℃; under 760.051 Torr; for 2h; Temperature; Solvent; Reagent/catalyst; | 100% |

| Conditions | Yield |
|---|---|
| With nickel(II) chloride dihydrate In acetonitrile at 80℃; Molecular sieve; Inert atmosphere; | 100% |
| With oxalyl dichloride; triethylamine In dimethyl sulfoxide; acetonitrile at 20℃; for 0.333333h; Reagent/catalyst; Swern Oxidation; | 100% |
| With zinc trifluoromethanesulfonate In toluene at 100℃; for 24h; | 99% |

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0) In benzene at 120℃; for 12h; Product distribution; other acyl cyanides, var. solvents, temp. and time; | 100% |
| With tris(dibenzylideneacetone)dipalladium(0) chloroform complex; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In toluene at 180℃; for 24h; Temperature; Glovebox; Inert atmosphere; Sealed tube; | 95 %Chromat. |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; N,N-dimethyl-formamide at 35℃; for 24h; Schlenk technique; Inert atmosphere; Irradiation; | 100% |
| With C60H48BP3Pd; potassium formate; [2.2.2]cryptande In tetrahydrofuran at 60℃; for 72h; Schlenk technique; Inert atmosphere; | 99% |
| With formic acid; Cyclohexanethiol; 10-phenyl-10H-phenothiazine; N-ethyl-N,N-diisopropylamine In acetonitrile at 20℃; under 2625.26 Torr; for 0.05h; Catalytic behavior; Reagent/catalyst; Time; Wavelength; Irradiation; Flow reactor; | 96% |
-
-
932-90-1, 622-31-1, 140461-24-1, 140461-25-2, 622-32-2
syn-benzaldehyde oxime

-
-
100-47-0
benzonitrile

| Conditions | Yield |
|---|---|
| With triethylamine; 2,4-Dichloro-5-nitropyrimidine In acetonitrile for 5h; Ambient temperature; | 100% |
| With 1,3,5-trichloro-2,4,6-triazine In N,N-dimethyl-formamide at 20℃; Beckmann rearrangement; | 100% |
| With oxalyl dichloride; Triphenylphosphine oxide In chloroform at 20℃; for 1h; | 99% |
-
-
55-21-0
benzamide

-
-
78303-22-7
chloro(tert-butyl)diethylamino(methylene)phosphorane

-
A
-
100-47-0
benzonitrile

| Conditions | Yield |
|---|---|
| In diethyl ether -10 dec C, then +20 deg C.; | A 70% B 100% C 85% |

-
-
149540-88-5
(E)-benzaldehyde O-pivaloyloxime

-
-
100-47-0
benzonitrile

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 25℃; Rate constant; Mechanism; variation of base/solvent system; | 100% |
| With diisobutylamine In acetonitrile at 20℃; for 10h; | 94 %Chromat. |
| With diisopropylamine In acetonitrile at 25℃; Kinetics; |
-
-
149540-89-6
C12H14(2)HNO2

-
-
100-47-0
benzonitrile

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 25℃; Rate constant; variation of base/solvent system; | 100% |

| Conditions | Yield |
|---|---|
| With sodium carbonate; palladium diacetate In 1-methyl-pyrrolidin-2-one; Hexadecane at 140℃; for 16h; Product distribution / selectivity; | 100% |
| With [Pd{C6H3(CH2CH2NH2)-4-OMe-5-κ2-C,N}(μ-Br)]2; potassium carbonate In N,N-dimethyl-formamide at 130℃; for 0.166667h; Microwave irradiation; | 94% |
| With sodium carbonate; palladium diacetate; tri-tert-butyl phosphine In 1-methyl-pyrrolidin-2-one; Hexadecane at 140℃; for 16h; Product distribution / selectivity; | 88% |
-
-
2042-37-7
o-cyanobromobenzene

-
-
7440-66-6
zinc

-
A
-
4341-02-0
biphenyl-2,2'-dicarbonitrile

-
B
-
100-47-0
benzonitrile

-
C
-
131379-17-4
2-cyanophenylzinc bromide

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid; cobalt(II) bromide; zinc dibromide In acetonitrile the mixt. in CH3CN was stirred at room temp., then arylbromide was added, stirred at room temp.; GC analysis; | A 0% B 0% C 100% |


-
-
10102-43-9
nitrogen(II) oxide

-
B
-
100-47-0
benzonitrile

| Conditions | Yield |
|---|---|
| With NaClO4; CH2Cl2 In dichloromethane room temp., 15 min; | A 80% B 100% |
| With CH2Cl2 In dichloromethane room temp., 15 min; | A n/a B 73% |


| Conditions | Yield |
|---|---|
| With sodium carbonate; palladium diacetate In 1-methyl-pyrrolidin-2-one; Hexadecane at 140℃; for 16h; Product distribution / selectivity; | 100% |
| With [Pd{C6H3(CH2CH2NH2)-4-OMe-5-κ2-C,N}(μ-Br)]2; potassium carbonate In N,N-dimethyl-formamide at 130℃; for 0.116667h; Microwave irradiation; | 95% |
| With 1-methyl-pyrrolidin-2-one; 1,1'-bis-(diphenylphosphino)ferrocene; palladium diacetate; sodium carbonate at 120℃; for 12h; Schlenk technique; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| With oxygen; sodium hydroxide In tetrahydrofuran; water at 20℃; for 3h; | 99% |
| With p-methoxybenzenetellurinic acid anhydride In dichloromethane for 0.5h; Ambient temperature; | 95% |
| With bis(4-methoxyphenyl)telluride; tetrabutylammonium acetate In water; acetonitrile electrolysis; | 95% |

| Conditions | Yield |
|---|---|
| With oxygen In acetone at 20℃; for 5.3h; Electrochemical reaction; | 99% |
| With aluminum oxide In N,N-dimethyl-formamide at 120℃; for 6h; Catalytic behavior; Reagent/catalyst; Inert atmosphere; | 99% |
| With water; potassium hydroxide at 25℃; pH=13.6; Electrochemical reaction; | 98% |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; oxygen In tert-Amyl alcohol at 130℃; under 3750.38 Torr; for 24h; Reagent/catalyst; Solvent; | 99% |
| With ammonia; oxygen In tert-Amyl alcohol; water at 100℃; under 3750.38 Torr; for 5h; Autoclave; High pressure; | 99% |
| With ammonia at 320℃; for 2h; Catalytic behavior; Reagent/catalyst; Temperature; Flow reactor; | 98% |

| Conditions | Yield |
|---|---|
| With ammonium formate In water at 20℃; for 6h; | 99% |
| With Triethoxysilane; C20H24N4Ni; sodium t-butanolate In toluene at 80℃; for 8h; Kumada Cross-Coupling; Inert atmosphere; Schlenk technique; | 83% |
| With cyclohexa-1,4-diene; 9-ethyl-N3,N3,N6,N6,-tetramethyl-9H-carbazole-3,6-diamine; N-ethyl-N,N-diisopropylamine In N,N-dimethyl acetamide at 23℃; for 0.8h; Inert atmosphere; UV-irradiation; Schlenk technique; | 83% |

| Conditions | Yield |
|---|---|
| With Palladium Nanoparticles with two shape-persistent covalent cages CC1' In N,N-dimethyl-formamide at 140℃; for 15h; Reagent/catalyst; Inert atmosphere; | 99% |
| With tetrabutylammomium bromide; copper(II) acetate monohydrate; potassium iodide; N,N`-dimethylethylenediamine In water at 20 - 140℃; Microwave irradiation; | 89% |
| With sodium carbonate In N,N-dimethyl-formamide at 110℃; for 20h; Catalytic behavior; Sealed tube; | 99 %Chromat. |

| Conditions | Yield |
|---|---|
| With sodium carbonate In N,N-dimethyl-formamide at 120℃; for 12h; Catalytic behavior; Reagent/catalyst; Solvent; Time; Temperature; Inert atmosphere; | 99% |
| With sodium carbonate In N,N-dimethyl-formamide at 120℃; for 3h; | 95% |
| With [Pd{C6H4(CH2N(CH2Ph)2)}(μ-Br)]2; tetrabutylammomium bromide; potassium carbonate In N,N-dimethyl-formamide at 130℃; for 0.0833333h; Microwave irradiation; | 92% |

| Conditions | Yield |
|---|---|
| With triethylamine In water at 25℃; for 1h; UV-irradiation; | 99% |
| With triethylamine In tetrahydrofuran at 20℃; for 12h; Irradiation; Inert atmosphere; Sealed tube; | 80.7% |
| With triethylamine; Lumogen F Orange 240 In N,N-dimethyl-formamide at 40℃; for 4h; Irradiation; | 98 %Chromat. |

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 24.84℃; under 760.051 Torr; for 96h; Inert atmosphere; UV-irradiation; Sealed tube; | 99% |

-
-
100-47-0
benzonitrile

| Conditions | Yield |
|---|---|
| With copper(l) iodide In dimethyl sulfoxide at 90℃; for 12h; | 99% |

| Conditions | Yield |
|---|---|
| With iron(III) chloride; 2,6-di-tert-butyl-4-methyl-phenol In dichloromethane; toluene at 20℃; for 0.0333333h; Reagent/catalyst; | 99% |
| With iron(III) chloride; 2,6-di-tert-butyl-4-methyl-phenol In toluene at 20℃; for 0.0333333h; Catalytic behavior; Reagent/catalyst; Solvent; Schlenk technique; | 64% |
-
-
18322-87-7, 63561-63-7
syn-O-(4-Chlorbenzoyl)-benzaldoxim

-
-
100-47-0
benzonitrile

| Conditions | Yield |
|---|---|
| With iron(III) chloride; 2,6-di-tert-butyl-4-methyl-phenol In dichloromethane; toluene at 20℃; for 0.05h; | 99% |

-
-
100-47-0
benzonitrile

| Conditions | Yield |
|---|---|
| With iron(III) chloride; 2,6-di-tert-butyl-4-methyl-phenol In dichloromethane; toluene at 20℃; for 0.05h; | 99% |

| Conditions | Yield |
|---|---|
| With tris(dibenzylideneacetone)dipalladium(0) chloroform complex; 1,1'-bis-(diphenylphosphino)ferrocene In various solvent(s) at 60℃; for 1h; | 98% |
| With tris(dibenzylideneacetone)dipalladium(0) chloroform complex; 1,1'-bis-(diphenylphosphino)ferrocene In various solvent(s) at 60℃; for 1h; other catalyst, other solvent, other temperature; | 98% |
| Stage #1: potassium cyanide; (E)-N1,N1-dimethyl-N2-(pyridin-2-ylmethylene)ethane-1,2-diamine; copper(I) oxide at 100℃; Stage #2: iodobenzene In N,N-dimethyl-formamide at 110℃; for 24h; Product distribution / selectivity; | 83% |
| Stage #1: potassium cyanide; copper(I) oxide; trans-N,N'-bis(pyridin-2-ylmethylene)cyclohexane-1,2-diamine at 100℃; Stage #2: iodobenzene In N,N-dimethyl-formamide at 110℃; for 24 - 48h; Product distribution / selectivity; | 73.7% |
| With copper(l) iodide; tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran for 1h; Substitution; Heating; |
-
-
79-46-9
2-nitropropane

-
-
80670-36-6
3-diazo-5-phenyl-3H-1,2,4-triazole

-
A
-
100-47-0
benzonitrile

-
B
-
127-06-0
acetone oxime

| Conditions | Yield |
|---|---|
| at 80℃; Yields of byproduct given; | A n/a B 98% |

-
-
21272-78-6
α-nitrobenzyl phenyl sulfone

-
A
-
77853-07-7
Benzenethiosulfonic acid; compound with ammonia

-
B
-
100-47-0
benzonitrile

| Conditions | Yield |
|---|---|
| With sulfur; ammonia at 100℃; for 4h; | A 98% B 70% |
-
-
55986-20-4
N-(p-tolylsulfonyl)dibenzylselenimide

-
-
2227-79-4
benzenecarbothioamide

-
A
-
1842-38-2
dibenzyl selenide

-
B
-
70-55-3
toluene-4-sulfonamide

-
C
-
100-47-0
benzonitrile

| Conditions | Yield |
|---|---|
| In methanol for 0.5h; Mechanism; Ambient temperature; | A 98% B 98% C 81% |

-
-
80503-58-8
4-hydroxy-4-octyl-3-phenylisoxazoline-5-one

-
A
-
333-60-8
2-oxodecanoic acid

-
B
-
100-47-0
benzonitrile

| Conditions | Yield |
|---|---|
| With 2,6-dichloro-benzonitrile In benzene for 3h; Heating; | A 98% B n/a |
-
-
100-47-0
benzonitrile

-
-
18039-42-4
5-Phenyl-1H-tetrazole

| Conditions | Yield |
|---|---|
| With sodium azide In dimethyl sulfoxide at 140℃; for 1h; Solvent; Time; Temperature; | 100% |
| With sodium azide In dimethyl sulfoxide at 120℃; for 0.166667h; Catalytic behavior; Solvent; Reagent/catalyst; Temperature; Green chemistry; | 100% |
| With sodium azide In N,N-dimethyl-formamide at 120℃; for 8h; Catalytic behavior; Reagent/catalyst; Solvent; Green chemistry; | 100% |

| Conditions | Yield |
|---|---|
| With ammonia; lanthanum(lll) triflate at 200℃; for 24h; in a stainless steel pressure vessel; | 100% |
| With samarium diiodide; hexan-1-amine at 80℃; for 3h; cyclotrimerization; | 96% |
| With samarium diiodide; hexan-1-amine at 80℃; for 3h; Product distribution; Further Variations:; Reagents; Temperatures; nitrile:amine mole ratio; cyclotrimerization; | 96% |

| Conditions | Yield |
|---|---|
| With water; nitrile hydratase from Rhodococcus rhodochrous J1 at 25℃; for 24h; K2HPO4-KH2PO4 puffer pH=8.0; | 100% |
| With manganese(IV) oxide; silica gel In 2,2,4-trimethylpentane for 2h; Heating; | 100% |
| With sodium hydroxide; trisodium tris(3-sulfophenyl)phosphine; water; chloro(1,5-cyclooctadiene)rhodium(I) dimer In ethyl acetate at 90℃; for 24h; pH=11.7; hydration; | 100% |

| Conditions | Yield |
|---|---|
| With pyridine; diammonium sulfide; triethylamine In water at 50℃; | 100% |
| With sodium hydrogensulfide; diethyl amine hydrochloride In 1,4-dioxane; water at 55℃; for 6h; | 98% |
| With diisopropyldithiophosphoric acid In methanol at 60℃; for 4h; | 97% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; hydroxylamine hydrochloride In methanol Heating; | 100% |
| With hydroxylamine In ethanol; water for 48h; Heating / reflux; | 100% |
| With hydroxylamine In ethanol; water Reflux; | 100% |

| Conditions | Yield |
|---|---|
| With water at 45℃; pH=7.2; Microbiological reaction; aq. buffer; | 100% |
| With potassium tert-butylate; water In isopropyl alcohol at 25℃; Inert atmosphere; | 100% |
| With benzene-1,2-dicarboxylic acid at 250℃; under 7600 Torr; for 0.25h; microwave irradiation; | 99% |

| Conditions | Yield |
|---|---|
| With lithium borohydride; 9-methoxy-9-BBN In diethyl ether at 25℃; for 5h; Product distribution; rate of reduction; | 100% |
| With borane N-ethyl-N-isopropylaniline complex In tetrahydrofuran for 0.1h; Heating; | 100% |
| With hydrogen; palladium In methanol at 20℃; for 432h; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: benzonitrile With woollins’ reagent In toluene for 4h; Heating; Stage #2: With water In toluene for 1h; | 100% |
| With selenium; sodium tetrahydroborate In pyridine; ethanol Heating; | 98% |
| Stage #1: benzonitrile With woollins’ reagent In toluene under 760.051 Torr; for 8h; Inert atmosphere; Schlenk technique; Reflux; Stage #2: With water In toluene at 90℃; under 760.051 Torr; for 1h; Inert atmosphere; Schlenk technique; Reflux; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl amine With n-butyllithium In diethyl ether; hexane at -20℃; for 0.166667h; Stage #2: benzonitrile at -20℃; for 1h; | 100% |

| Conditions | Yield |
|---|---|
| With phenyllithium In diethyl ether for 2.5h; Heating; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-indoline; benzonitrile With aluminum (III) chloride; water; boron trichloride In dichloromethane; toluene at 110℃; Stage #2: With hydrogenchloride; water In dichloromethane; toluene at 80℃; for 1h; | 100% |
| Stage #1: 1-indoline; benzonitrile With aluminum (III) chloride; boron trichloride In dichloromethane; toluene at 0 - 110℃; Stage #2: With hydrogenchloride; water at 80℃; for 1h; | 100% |
| With aluminum (III) chloride; boron trichloride In toluene Friedel-Crafts Acylation; Inert atmosphere; Reflux; | 84% |

| Conditions | Yield |
|---|---|
| (η3-allyl)(η5-pentamethylcyclopentadienyl)cobalt In hexane at 20℃; for 72h; | 100% |
| With aluminium trichloride 1.) CH2Cl2, -85 deg C, 2.) -50 deg C; Yield given. Multistep reaction; |
-
-
100-47-0
benzonitrile

-
-
20046-60-0, 38774-32-2
3β-acetoxy-16β,17β-epoxymethyleneandrost-5-ene

| Conditions | Yield |
|---|---|
| With tetrafluoroboric acid diethyl ether In diethyl ether; dichloromethane for 30h; Ambient temperature; | 100% |
-
-
100-47-0
benzonitrile

-
-
3287-99-8, 39110-74-2
benzylamine hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride; hydrogen In propan-1-ol; water at 60℃; under 375.038 Torr; for 18h; Flow reactor; | 100% |
| Stage #1: benzonitrile With [2,6-η6:η1-bis(2,4,6-trimethylphenyl)phenylthiolato]triethylphosphineruthenium(II)tetrakis[3,5-bis(trifluoromethyl)phenyl]borate; diethylphenylsilane at 20℃; for 18h; Glovebox; Inert atmosphere; Stage #2: With hydrogenchloride In diethyl ether at 20℃; for 1h; Glovebox; Inert atmosphere; | 99% |
| Stage #1: benzonitrile With hydrogen at 130℃; under 750.075 Torr; for 6h; Stage #2: Acidic conditions; chemoselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: benzonitrile With Li(2,2,6,6-tetramethylpiperidide)*Al(iBu)3 In tetrahydrofuran; hexane at -78℃; for 2h; Stage #2: With iodine In tetrahydrofuran; hexane at 0℃; for 1h; | 100% |
| Stage #1: benzonitrile With Li(2,2,6,6-tetramethylpiperidide)*Al(iBu)3 In tetrahydrofuran; hexane at -78℃; for 2h; Stage #2: With iodine In tetrahydrofuran; hexane at -78 - 0℃; for 1h; | 100% |
| Stage #1: benzonitrile With 2,2,6,6-tetramethylpiperidin-4-yl heptanoate; n-butyllithium; dichloro(N,N,N’,N‘-tetramethylethylenediamine)zinc In tetrahydrofuran; hexane at 0 - 20℃; Inert atmosphere; Stage #2: With iodine In tetrahydrofuran; hexane Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With hydroxylamine In ethanol for 1h; Heating / reflux; | 100% |
| With hydroxylamine In ethanol for 1h; Reflux; | 100% |
| With hydroxylamine hydrochloride; sodium carbonate In ethanol for 17h; Reflux; | 100% |
-
-
635-46-1
1,2,3,4-tetrahydroisoquinoline

-
-
100-47-0
benzonitrile

-
-
28748-92-7
phenyl(1,2,3,4-tetrahydroquinolin-8-yl)methanone

| Conditions | Yield |
|---|---|
| Stage #1: 1,2,3,4-tetrahydroisoquinoline; benzonitrile With aluminum (III) chloride; water; boron trichloride In dichloromethane; toluene at 110℃; Stage #2: With hydrogenchloride; water In dichloromethane; toluene at 80℃; for 1h; | 100% |
| Stage #1: 1,2,3,4-tetrahydroisoquinoline; benzonitrile With aluminum (III) chloride; boron trichloride In dichloromethane; toluene at 0 - 110℃; Stage #2: With hydrogenchloride; water at 80℃; for 1h; | 100% |
| With aluminium trichloride; boron trichloride In 1,2-dichloro-ethane Acylation; |

| Conditions | Yield |
|---|---|
| Stage #1: (2-picolyl)trimethylsilane With lithium diisopropyl amide In tetrahydrofuran at -80℃; for 1h; Stage #2: benzonitrile In tetrahydrofuran at -80 - 20℃; for 3h; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: N,N-dimethyl(trimethylsilyl)acetamide With n-butyllithium In tetrahydrofuran; hexane at -80℃; for 1h; Stage #2: benzonitrile In tetrahydrofuran at -80 - 20℃; for 3h; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: benzonitrile; ethyl(diphenyl)phosphine borane With N,N,N,N,N,N-hexamethylphosphoric triamide; sec.-butyllithium In tetrahydrofuran at -90℃; for 0.5h; Stage #2: allyl bromide In tetrahydrofuran at -90℃; for 2h; | 100% |


| Conditions | Yield |
|---|---|
| Stage #1: benzonitrile; ethyl(diphenyl)phosphine borane With N,N,N,N,N,N-hexamethylphosphoric triamide; sec.-butyllithium In tetrahydrofuran at -90℃; for 0.5h; Stage #2: methyl iodide In tetrahydrofuran at -90℃; for 2h; | 100% |

Benzonitrile History
Benzonitrile Consensus Reports
Benzonitrile Standards and Recommendations
Benzonitrile Specification
1. Introduction of Benzonitrile
The Benzonitrile is an organic compound with the formula C7H5N. The IUPAC name of this chemical is benzonitrile. With the CAS registry number 100-47-0, it is also named as benzoic acid nitrile. The product's categories are Industrial/Fine Chemicals; Intermediates; Organics. Besides, it is colourless liquid, which should be stored in a cool and well-ventilated place.
2. Properties of Benzonitrile
(1)ACD/LogP: 1.58; (2)ACD/LogD (pH 5.5): 1.584; (3)ACD/LogD (pH 7.4): 1.584; (4)ACD/BCF (pH 5.5): 9.413; (5)ACD/BCF (pH 7.4): 9.413; (6)ACD/KOC (pH 5.5): 173.222; (7)ACD/KOC (pH 7.4): 173.222; (8)#H bond acceptors: 1; (9)Polar Surface Area: 23.79 Å2; (10)Index of Refraction: 1.539; (11)Molar Refractivity: 31.32 cm3; (12)Molar Volume: 99.952 cm3; (13)Polarizability: 12.416×10-24cm3; (14)Surface Tension: 41.066 dyne/cm; (15)Density: 1.032 g/cm3; (16)Flash Point: 71.667 °C; (17)Enthalpy of Vaporization: 42.73 kJ/mol; (18)Boiling Point: 191.099 °C at 760 mmHg; (19)Vapour Pressure: 0.524 mmHg at 25°C.
3. Structure Descriptors of Benzonitrile
(1)SMILES: c1ccc(cc1)C#N
(2)InChI: InChI=1/C7H5N/c8-6-7-4-2-1-3-5-7/h1-5H
(3)InChIKey: JFDZBHWFFUWGJE-UHFFFAOYAY
(4)Std. InChI: InChI=1S/C7H5N/c8-6-7-4-2-1-3-5-7/h1-5H
(5)Std. InChIKey: JFDZBHWFFUWGJE-UHFFFAOYSA-N
4. Toxicity of Benzonitrile
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| cat | LD50 | oral | 800mg/kg (800mg/kg) | BEHAVIORAL: TREMOR BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Food and Cosmetics Toxicology. Vol. 17, Pg. 723, 1979. |
| frog | LDLo | subcutaneous | 1700mg/kg (1700mg/kg) | PERIPHERAL NERVE AND SENSATION: SPASTIC PARALYSIS WITH OR WITHOUT SENSORY CHANGE LUNGS, THORAX, OR RESPIRATION: DYSPNEA CARDIAC: OTHER CHANGES | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 5, Pg. 161, 1899. |
| mammal (species unspecified) | LD50 | oral | 800mg/kg (800mg/kg) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 32(10), Pg. 25, 1988. | |
| mouse | LC50 | inhalation | 1800mg/m3 (1800mg/m3) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 21(6), Pg. 34, 1977. | |
| mouse | LD50 | intraperitoneal | 400mg/kg (400mg/kg) | Food and Cosmetics Toxicology. Vol. 17, Pg. 723, 1979. | |
| mouse | LD50 | oral | 971mg/kg (971mg/kg) | Nippon Eiseigaku Zasshi. Japanese Journal of Hygiene. Vol. 39, Pg. 423, 1984. | |
| pigeon | LDLo | subcutaneous | 500mg/kg (500mg/kg) | Food and Cosmetics Toxicology. Vol. 17, Pg. 723, 1979. | |
| rabbit | LD50 | intraperitoneal | 1250mg/kg (1250mg/kg) | Food and Cosmetics Toxicology. Vol. 17, Pg. 723, 1979. | |
| rabbit | LD50 | oral | 800mg/kg (800mg/kg) | BEHAVIORAL: TREMOR BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Food and Cosmetics Toxicology. Vol. 17, Pg. 723, 1979. |
| rabbit | LD50 | skin | 1250mg/kg (1250mg/kg) | Food and Cosmetics Toxicology. Vol. 17, Pg. 723, 1979. | |
| rabbit | LDLo | subcutaneous | 200mg/kg (200mg/kg) | PERIPHERAL NERVE AND SENSATION: SPASTIC PARALYSIS WITH OR WITHOUT SENSORY CHANGE BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 5, Pg. 161, 1899. |
| rat | LCLo | inhalation | 950ppm/8H (950ppm) | LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Aerospace Medical Research Laboratory Report. Vol. TR-74-78, Pg. 1974, |
| rat | LD50 | intraperitoneal | 740mg/kg (740mg/kg) | SENSE ORGANS AND SPECIAL SENSES: PTOSIS: EYE BEHAVIORAL: MUSCLE WEAKNESS LUNGS, THORAX, OR RESPIRATION: ACUTE PULMONARY EDEMA | Annales Pharmaceutiques Francaises. Vol. 48, Pg. 23, 1990. |
| rat | LD50 | skin | 1200mg/kg (1200mg/kg) | Aerospace Medical Research Laboratory Report. Vol. TR-74-78, Pg. 1974, | |
| rat | LDLo | oral | 720mg/kg (720mg/kg) | Aerospace Medical Research Laboratory Report. Vol. TR-74-78, Pg. 1974, |
5. Safety Information of Benzonitrile
Hazard Symbols:
 Xn
XnRisk Codes:
R21/22:Harmful in contact with skin and if swallowed.
Safety Description:
S23:Do not breathe vapour.
6. Preparation of Benzonitrile
Benzonitrile can be prepared by benzaldehyde. This reaction will need reagent TMSA, catalyst ZnCl2 and solvent CHCl3. The reaction time is 4 hours at ambient temperature. The yield is about 2%.
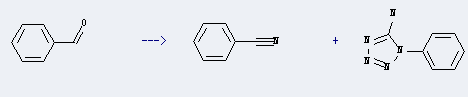
7. Use of Benzonitrile
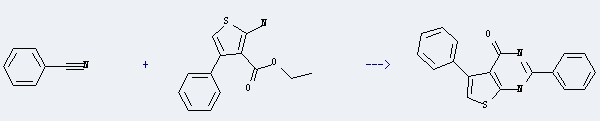
Benzonitrile can be used to produce 2,5-diphenyl-1H-thieno[2,3-d]pyrimidin-4-one at ambient temperature. It will need reagent HCl. The yield is about 35.5%.
The Benzonitrile is a useful solvent and a versatile precursor to many derivatives. It reacts with amines to afford N-substituted benzamides after hydrolysis. It is a precursor to Ph2C=NH (b.p. 151 °C, 8 mm Hg) via reaction with phenylmagnesium bromide followed by methanolysis. Benzonitrile can form coordination complexes with late transition metals that are both soluble in organic solvents and conveniently labile. The benzonitrile ligands are readily displaced by stronger ligands, making benzonitrile complexes useful synthetic intermediates.
8. Other details of Benzonitrile
When you are using Benzonitrile, please be cautious about it as the following:
It is harmful in contact with skin and if swallowed. When you are using it, do not breathe gas/fumes/vapour/spray (appropriate wording to be specified by the manufacturer).
Related Products
- Benzonitrile
- Benzonitrile, 2,4-dichloro-6-methyl-
- Benzonitrile, 2,4-dimethoxy-6-methyl-
- Benzonitrile, 2,6-dinitro-
- Benzonitrile, 2-[(6-chloro-3,4-dihydro-2,4-dioxo-1(2H)-pyrimidinyl)methyl]-
- Benzonitrile, 2-amino-4-nitro-
- Benzonitrile, 2-chloro-3-formyl-
- Benzonitrile, 2-chloro-4-fluoro-3-methyl-
- Benzonitrile, 2-chloro-6-methoxy-
- Benzonitrile, 2-ethyl-
- 100471-46-3
- 10047-28-6
- 10047-33-3
- 1004-74-6
- 1004-75-7
- 1004-76-8
- 1004781-36-5
- 100-48-1
- 100481-09-2
- 10048-13-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View