-
Name
(S)-(-)-N,N-Dimethyl-3-hydroxy-3-(2-thienyl)propanamine
- EINECS 603-565-7
- CAS No. 132335-44-5
- Article Data47
- CAS DataBase
- Density 1.111 g/cm3
- Solubility
- Melting Point 78-80 °C
- Formula C9H15NOS
- Boiling Point 290.119 °C at 760 mmHg
- Molecular Weight 185.29
- Flash Point 129.259 °C
- Transport Information
- Appearance
- Safety 26-36/37/39
- Risk Codes 36/37/38
-
Molecular Structure
- Hazard Symbols
- Synonyms 2-Thiophenemethanol,a-[2-(dimethylamino)ethyl]-, (S)-;(1S)-3-(Dimethylamino)-1-(2-thienyl)-1-propanol;
- PSA 51.71000
- LogP 1.73320
Synthetic route
-
-
287737-72-8
(S)-N,N-dimethyl-N-[3-hydroxy-3-(2-thienyl)propyl]-ammonium (S)-mandelate

-
-
132335-44-5
(S)-3-dimethylamino-1-(2-thienyl)propan-1-ol

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water for 0.5h; pH=11 - 12; | 95% |
| With ammonium hydroxide In tert-butyl methyl ether | 92.1% |
| With sodium hydroxide In tert-butyl methyl ether; water at 20℃; for 0.5h; pH=9; |
-
-
5424-47-5
3-(dimethylamino)-1-(thiophen-2-yl)propan-1-one hydrochloride

-
-
132335-44-5
(S)-3-dimethylamino-1-(2-thienyl)propan-1-ol

| Conditions | Yield |
|---|---|
| With glucose dehydrogenase; D-glucose; Rhodosporidium toruloides carbonyl reductase 9 from Escherichia coli; NADPH; sodium hydroxide In aq. phosphate buffer at 30℃; for 4h; pH=7; Kinetics; Temperature; Enzymatic reaction; enantioselective reaction; | 92.1% |
| With hydrogen; sodium hydrogencarbonate; (2R,4R)-4-(dicyclohexylphosphino)-2-(diphenylphosphino-methyl)-N-methyl-aminocarbonyl-pyrrolidine; di-μ-chloro-bis(1,5-cyclooctadiene)dirhodium In methanol; water at 30℃; under 75007.5 Torr; for 20h; | |
| Multi-step reaction with 3 steps 1: sodium tetrahydroborate; sodium hydroxide / water / 7 h / 20 - 71 °C 2: toluene / 0.5 h / 80 °C 3: sodium hydroxide / water View Scheme |
-
-
13636-02-7, 132335-44-5, 132335-49-0
3-(N,N-dimethylamino)-1-(thien-2-yl)propan-1-ol

-
-
132335-44-5
(S)-3-dimethylamino-1-(2-thienyl)propan-1-ol

| Conditions | Yield |
|---|---|
| Stage #1: 3-(N,N-dimethylamino)-1-(thien-2-yl)propan-1-ol With tert-butyl methyl ether; (S)-Mandelic acid Stage #2: With sodium hydroxide | 92% |
| With (S)-Mandelic acid In methanol; toluene at 20 - 95℃; for 1.5h; pH=1 - 12; | 34% |
| Multi-step reaction with 2 steps 1: toluene / 0.5 h / 80 °C 2: sodium hydroxide / water View Scheme |

-
-
132335-44-5
(S)-3-dimethylamino-1-(2-thienyl)propan-1-ol

| Conditions | Yield |
|---|---|
| With methanol at 80℃; for 20h; Concentration; | 92% |
-
-
124-40-3
dimethyl amine

-
-
164071-56-1
(S)-3-chloro-1-(thiophen-2-yl)propan-1-ol

-
-
132335-44-5
(S)-3-dimethylamino-1-(2-thienyl)propan-1-ol

| Conditions | Yield |
|---|---|
| With potassium iodide In methanol; water at 80℃; for 8h; | 89% |
-
-
13196-35-5
3-(dimethylamino)-1-(thiophen-2-yl)propan-1-one

-
-
132335-44-5
(S)-3-dimethylamino-1-(2-thienyl)propan-1-ol

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; hydrogen; RuCl2[(R)-(DM-BINAP)][(R)-DAIPEN] In isopropyl alcohol; tert-butyl alcohol at 28℃; for 6h; | 80% |
| With hydrogen; (2R,2'R)-bis(diphenylphosphino)-(1R,1'R)-dicyclopentane; 1,1-dimethylethylenediamine; [RuCl2(dmf)n]; sodium t-butanolate In ethanol; dichloromethane at 20℃; under 5320 Torr; for 15h; | |
| With RuCl2(1,1'-bis(diphenyphosphino)ferrocene)[(1S,1'S)-6,6'-dibromo-1,1'-biisoindoline]; potassium tert-butylate; hydrogen In propan-1-ol at 20℃; under 38002.6 Torr; for 30h; Inert atmosphere; Autoclave; optical yield given as %ee; enantioselective reaction; |

-
-
132335-44-5
(S)-3-dimethylamino-1-(2-thienyl)propan-1-ol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: phosphoric acid / water / 18 h / 40 - 45 °C 2: ethanol; tert-butyl methyl ether / 50 °C 3: ammonium hydroxide / tert-butyl methyl ether View Scheme |

-
-
132335-44-5
(S)-3-dimethylamino-1-(2-thienyl)propan-1-ol

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 2h; Alkaline conditions; | 26.4 g |

-
-
132335-44-5
(S)-3-dimethylamino-1-(2-thienyl)propan-1-ol

| Conditions | Yield |
|---|---|
| With water; sodium hydroxide | 30.2 g |
-
-
34772-98-0
3-dimethylamino-1-thiophen-2-ylpropenone

-
-
132335-44-5
(S)-3-dimethylamino-1-(2-thienyl)propan-1-ol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: lithium aluminium tetrahydride / tetrahydrofuran / 8 h / Reflux 2: ethanol; tert-butyl methyl ether / 1 h / Reflux 3: sodium hydroxide / water / 0.5 h / pH 11 - 12 View Scheme |

-
A
-
132335-49-0
α-[2-(dimethylamino)ethyl](thiophene-2-yl)methanol

-
B
-
132335-44-5
(S)-3-dimethylamino-1-(2-thienyl)propan-1-ol

| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethyl acetate at 20℃; for 1h; | A n/a B 65 mg |
-
-
321-38-0
1-Fluoronaphthalene

-
-
132335-44-5
(S)-3-dimethylamino-1-(2-thienyl)propan-1-ol

-
-
132335-46-7
N-methyl-(S)-duloxetine

| Conditions | Yield |
|---|---|
| Stage #1: (S)-3-dimethylamino-1-(2-thienyl)propan-1-ol With sodium hydride In dimethyl sulfoxide at 60℃; for 1.16667h; Stage #2: 1-Fluoronaphthalene In dimethyl sulfoxide at 60℃; for 24h; Solvent; Reagent/catalyst; | 93.9% |
| Stage #1: (S)-3-dimethylamino-1-(2-thienyl)propan-1-ol With sodium hydride In dimethyl sulfoxide; mineral oil at 20℃; for 0.5h; Stage #2: With Potassium benzoate In dimethyl sulfoxide; mineral oil for 0.5h; Stage #3: 1-Fluoronaphthalene In dimethyl sulfoxide; mineral oil at 65℃; for 8h; | 92% |
| Stage #1: (S)-3-dimethylamino-1-(2-thienyl)propan-1-ol With sodium hydride In dimethyl sulfoxide; mineral oil at 20℃; for 0.5h; Stage #2: 1-Fluoronaphthalene In dimethyl sulfoxide; mineral oil at 40 - 50℃; for 8h; | 90% |
-
-
132335-44-5
(S)-3-dimethylamino-1-(2-thienyl)propan-1-ol

-
-
75-36-5
acetyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In chloroform at 5 - 20℃; for 2.16667h; | 92% |
| With triethylamine In toluene at 25℃; for 6.5h; | 60 g |
-
-
321-38-0
1-Fluoronaphthalene

-
-
132335-44-5
(S)-3-dimethylamino-1-(2-thienyl)propan-1-ol

| Conditions | Yield |
|---|---|
| Stage #1: (S)-3-dimethylamino-1-(2-thienyl)propan-1-ol With potassium hydroxide; 18-crown-6 ether In dimethyl sulfoxide at 60℃; for 1h; Stage #2: 1-Fluoronaphthalene In dimethyl sulfoxide for 2h; Stage #3: With oxalic acid In ethyl acetate for 1h; | 88% |
-
-
943830-74-8
4-fluorobenzo[1,3]dioxolane

-
-
132335-44-5
(S)-3-dimethylamino-1-(2-thienyl)propan-1-ol

| Conditions | Yield |
|---|---|
| Stage #1: (S)-3-dimethylamino-1-(2-thienyl)propan-1-ol With sodium hydride In dimethyl sulfoxide; mineral oil at 60℃; for 0.5h; Stage #2: With Potassium benzoate In dimethyl sulfoxide; mineral oil for 0.333333h; Stage #3: 4-fluorobenzo[1,3]dioxolane In dimethyl sulfoxide; mineral oil at 60℃; for 8h; | 82% |
| Stage #1: (S)-3-dimethylamino-1-(2-thienyl)propan-1-ol With Potassium benzoate; sodium hydride In dimethyl sulfoxide at 60℃; for 0.5h; Stage #2: 4-fluorobenzo[1,3]dioxolane In dimethyl sulfoxide at 60℃; for 8h; | 4.8 g |
-
-
132335-44-5
(S)-3-dimethylamino-1-(2-thienyl)propan-1-ol

-
-
543-27-1
isobutyl chloroformate

-
-
116539-55-0
(S)-3-methylamino-1-(2-thienyl)-1-propanol

| Conditions | Yield |
|---|---|
| Stage #1: (S)-3-dimethylamino-1-(2-thienyl)propan-1-ol; isobutyl chloroformate With triethylamine In toluene at 20 - 30℃; for 6h; Stage #2: With potassium hydroxide In methanol at 75℃; for 3.5h; Heating / reflux; | 79.6% |

-
-
132335-44-5
(S)-3-dimethylamino-1-(2-thienyl)propan-1-ol

| Conditions | Yield |
|---|---|
| With copper(l) iodide; N1, N2-diphenethyloxalamide; sodium t-butanolate In 1,4-dioxane for 24h; Schlenk technique; Inert atmosphere; Molecular sieve; Heating; | 70% |
-
-
90-11-9
1-Bromonaphthalene

-
-
132335-44-5
(S)-3-dimethylamino-1-(2-thienyl)propan-1-ol

-
-
132335-46-7
N-methyl-(S)-duloxetine

| Conditions | Yield |
|---|---|
| With copper(l) iodide; N1, N2-diphenethyloxalamide; sodium t-butanolate In 1,4-dioxane for 24h; Schlenk technique; Inert atmosphere; Molecular sieve; Heating; | 65% |
-
-
321-38-0
1-Fluoronaphthalene

-
-
144-62-7
oxalic acid

-
-
132335-44-5
(S)-3-dimethylamino-1-(2-thienyl)propan-1-ol

-
-
132335-47-8
(S)-N,N-dimethyl-3-(1-naphthyloxy)-3-(2-thienyl)propanamine oxalate

| Conditions | Yield |
|---|---|
| Stage #1: 1-Fluoronaphthalene; (S)-3-dimethylamino-1-(2-thienyl)propan-1-ol With tetrabutylammomium bromide; sodium hydroxide In dimethyl sulfoxide at 50 - 65℃; Stage #2: oxalic acid In toluene at 55 - 60℃; for 1h; optical yield given as %ee; enantioselective reaction; | 59.9% |
| Stage #1: 1-Fluoronaphthalene; (S)-3-dimethylamino-1-(2-thienyl)propan-1-ol With potassium tert-butylate at 90 - 100℃; Stage #2: oxalic acid In methanol at 0 - 30℃; |

-
-
321-38-0
1-Fluoronaphthalene

-
-
132335-44-5
(S)-3-dimethylamino-1-(2-thienyl)propan-1-ol

-
-
136434-34-9
duloxetine hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: (S)-3-dimethylamino-1-(2-thienyl)propan-1-ol With sodium hydride In dimethyl sulfoxide at 20℃; for 0.5h; Inert atmosphere; Stage #2: 1-Fluoronaphthalene In dimethyl sulfoxide at 60℃; Inert atmosphere; | 56% |
| Multi-step reaction with 2 steps 1.1: sodium hydride / mineral oil; dimethyl sulfoxide / 0.5 h / 20 °C 1.2: 8 h / 40 - 50 °C 2.1: carbonochloridic acid 1-chloro-ethyl ester / dichloromethane / 3 h / 0 - 5 °C / Reflux 2.2: 1 h / 50 °C View Scheme |
-
-
321-38-0
1-Fluoronaphthalene

-
-
132335-44-5
(S)-3-dimethylamino-1-(2-thienyl)propan-1-ol

-
-
132335-47-8
(S)-N,N-dimethyl-3-(1-naphthyloxy)-3-(2-thienyl)propanamine oxalate

| Conditions | Yield |
|---|---|
| With sodium hydride 1) DMSO, 25 deg C, 25 min 2) 48-72 h, 45-50 deg C; Yield given. Multistep reaction; |
-
-
132335-44-5
(S)-3-dimethylamino-1-(2-thienyl)propan-1-ol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1) NaH / 1) DMSO, 25 deg C, 25 min 2) 48-72 h, 45-50 deg C 2: 1) ClCO2CH2CCl3, Et3N, HCl 2) Zn-dust, 2.5percent formic acid / 1) toluene, 30 min, 25 deg C 2) DMF View Scheme | |
| Multi-step reaction with 3 steps 1.1: sodium hydride / dimethyl sulfoxide; mineral oil / 0.25 h / 25 °C 1.2: 0.25 h / 25 - 30 °C 1.3: 48 h / 25 - 30 °C 2.1: N-ethyl-N,N-diisopropylamine / toluene / 0.17 h 2.2: Reflux 3.1: potassium hydroxide / polyethylene glycol-400 / 16 h / 65 - 70 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: sodium hydride / mineral oil; dimethyl sulfoxide / 0.5 h / 20 °C 1.2: 0.5 h 1.3: 8 h / 65 °C 2.1: N-ethyl-N,N-diisopropylamine / toluene / 0.17 h / 45 °C 2.2: 2 h / 55 °C 3.1: sodium hydroxide / water; dimethyl sulfoxide / 8 h / 45 - 55 °C / Alkaline conditions View Scheme | |
| Multi-step reaction with 2 steps 1: sodium hydride 2: potassium hydroxide / ethanol; toluene / 2 h / 85 - 100 °C View Scheme |
-
-
132335-44-5
(S)-3-dimethylamino-1-(2-thienyl)propan-1-ol

-
-
1885-14-9
phenyl chloroformate

-
-
116539-55-0
(S)-3-methylamino-1-(2-thienyl)-1-propanol

| Conditions | Yield |
|---|---|
| Stage #1: (S)-3-dimethylamino-1-(2-thienyl)propan-1-ol; phenyl chloroformate With triethylamine In tert-butyl methyl ether at -5 - 20℃; for 4h; Stage #2: With potassium hydroxide In methanol; tert-butyl methyl ether for 2h; Heating / reflux; |
-
-
132335-44-5
(S)-3-dimethylamino-1-(2-thienyl)propan-1-ol

-
-
1885-14-9
phenyl chloroformate

-
-
625853-19-2
phenyl (RS)-N-methyl-N-[3-phenyloxycarbonyloxy-3-(thien-2-yl)propyl]carbamate

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 5 - 20℃; for 2h; |
-
-
321-38-0
1-Fluoronaphthalene

-
-
582-25-2
Potassium benzoate

-
-
132335-44-5
(S)-3-dimethylamino-1-(2-thienyl)propan-1-ol

-
-
161005-84-1
(S)-(+)-N,N-dimethyl-3-(1-naphthalenyloxy)-3-(2-thienyl)propanamine, phosphoric acid salt

| Conditions | Yield |
|---|---|
| With phosphoric acid In hexane; water; dimethyl sulfoxide; mineral oil |

-
-
321-38-0
1-Fluoronaphthalene

-
-
132335-44-5
(S)-3-dimethylamino-1-(2-thienyl)propan-1-ol

| Conditions | Yield |
|---|---|
| With potassium hydroxide In 1,2-dimethoxyethane at 60℃; for 8h; Product distribution / selectivity; | |
| With potassium hydroxide In Tetraethylene glycol dimethyl ether at 60℃; for 8h; Product distribution / selectivity; | |
| With potassium hydroxide In diethylene glycol dimethyl ether at 60℃; for 8h; Product distribution / selectivity; | |
| With potassium hydroxide In Triethylene glycol dimethyl ether at 60℃; for 8h; Product distribution / selectivity; |
-
-
321-38-0
1-Fluoronaphthalene

-
-
132335-44-5
(S)-3-dimethylamino-1-(2-thienyl)propan-1-ol

-
A
-
116539-60-7
(R)-Duloxetine

-
B
-
116539-58-3, 116539-59-4, 116539-60-7, 116817-13-1
Duloxetine

| Conditions | Yield |
|---|---|
| With potassium hydroxide In dimethyl sulfoxide at 60℃; for 8h; Product distribution / selectivity; |

-
-
321-38-0
1-Fluoronaphthalene

-
-
132335-44-5
(S)-3-dimethylamino-1-(2-thienyl)propan-1-ol

-
-
161005-84-1
(S)-(+)-N,N-dimethyl-3-(1-naphthalenyloxy)-3-(2-thienyl)propanamine, phosphoric acid salt

| Conditions | Yield |
|---|---|
| Stage #1: 1-Fluoronaphthalene; (S)-3-dimethylamino-1-(2-thienyl)propan-1-ol With potassium hydroxide In diethylene glycol dimethyl ether at 120℃; for 3 - 6h; Stage #2: With phosphoric acid In water; ethyl acetate for 1.08333 - 1.25h; | |
| Stage #1: (S)-3-dimethylamino-1-(2-thienyl)propan-1-ol With sodium hydride In dimethyl sulfoxide at 20℃; for 2h; Stage #2: 1-Fluoronaphthalene With potassium p-methyl benzoate In dimethyl sulfoxide at 20 - 65℃; for 5h; Stage #3: With phosphoric acid In ethyl acetate for 0.5h; pH=2; |
(S)-(-)-N,N-Dimethyl-3-hydroxy-3-(2-thienyl)propanamine Chemical Properties
Molecule structure of (S)-(-)-N,N-Dimethyl-3-hydroxy-3-(2-thienyl)propanamine (CAS NO.132335-44-5):
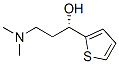
Molecular Weight: 185.2865 g/mol
Molecular Formula: C9H15NOS
Density: 1.111 g/cm3
Melting Point: 78-80 °C
Boiling Point: 290.119 °C at 760 mmHg
Flash Point: 129.259 °C
Index of Refraction: 1.553
Molar Refractivity: 53.344 cm3
Molar Volume: 166.758 cm3
Polarizability: 21.147×10-24 cm3
Surface Tension: 43.177 dyne/cm
Enthalpy of Vaporization: 55.918 kJ/mol
Vapour Pressure: 0.001 mmHg at 25 °C
InChI: InChI=1/C9H15NOS/c1-10(2)6-5-8(11)9-4-3-7-12-9/h3-4,7-8,11H,5-6H2,1-2H3/t8-/m0/s1 Copy
InChIKey: XWCNSHMHUZCRLN-QMMMGPOBBC
Product Categories of (S)-(-)-N,N-Dimethyl-3-hydroxy-3-(2-thienyl)propanamine (CAS NO.132335-44-5): Chemical intermediate for Duloxetine Hydrochloride
(S)-(-)-N,N-Dimethyl-3-hydroxy-3-(2-thienyl)propanamine Specification
(S)-(-)-N,N-Dimethyl-3-hydroxy-3-(2-thienyl)propanamine (CAS NO.132335-44-5) is also named as (1S)-3-(Dimethylamino)-1-(2-thienyl)-1-propanol ; (1S)-3-(Dimethylamino)-1-(2-thienyl)propan-1-ol ; (1S)-3-(dimethylamino)-1-(thiophen-2-yl)propan-1-ol ; 2-thiophenemethanol, alpha-[2-(dimethylamino)ethyl]-, (alphaS)- .
Related Products
- (S)-(-)-N-(5-Nitro-2-pyridyl)alaninol
- (S)-(-)-N,N-Dimethyl-1-ferrocenylethylamine
- (S)-(-)-N,N-Dimethyl-3-hydroxy-3-(2-thienyl)propanamine
- (S)-(-)-N-Benzyl-1-phenylethylamine
- (S)-(-)-N-Isopropyl-1-phenylethylamine hydrochloride
- (S)-(-)-N-Methyl-1-phenylethylamine
- 132335-46-7
- 132335-47-8
- 132336-37-9
- 1323-38-2
- 132338-80-8
- 1323-39-3
- 132339-37-8
- 132342-55-3
- 1323-42-8
- 132351-58-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View