-
Name
1,1,2-Trichlorotrifluoroethane
- EINECS 200-936-1
- CAS No. 76-13-1
- Article Data75
- CAS DataBase
- Density 1.67 g/cm3
- Solubility insoluble in water
- Melting Point -35 °C(lit.)
- Formula C2Cl3F3
- Boiling Point 50.9 °C at 760 mmHg
- Molecular Weight 187.376
- Flash Point 195°C
- Transport Information UN 3082 9/PG 3
- Appearance Colorless liquid with a sweet, ether-like odor
- Safety 59-61-45-36/37
- Risk Codes 52/53-59-39/23/24/25
-
Molecular Structure
-
Hazard Symbols
 N,
N, Xi,
Xi, T
T
- Synonyms 1,1,2-Trichloro-1,2,2-trifluoroethane;Arcton 113;Arklone P;Asahifron 113;CFC 113;Daiflon 113;Delifrene 113;Delifrene LS;FC 113;FKW 113;Fluorocarbon 113;Forane 113;Freon 113;Freon 113TR-T;Freon TF;Freon TS;Fridohna;Frigen 113;Frigen 113A;Frigen 113TR-N;Fron 113;Fronsolve113;Genetron 113;Halon 113;Isceon 113;Khladon 113;Ledon113;R 113;Refrigerant 113;Refrigerant R 113;
- PSA 0.00000
- LogP 2.91890
Synthetic route

| Conditions | Yield |
|---|---|
| With aluminum(III) fluoride; hydrogen fluoride at 120℃; under 11251.1 Torr; for 10h; Reagent/catalyst; Temperature; Autoclave; | 97.06% |
| With CFC-112a; antimonypentachloride; fluorine at 32℃; unter vermindertem Druck; | |
| With antimonypentachloride; antimony(III) fluoride at 48℃; |
-
-
7740-58-1
1,2,2-trifluorodichloroethanesulfonyl chloride

-
A
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

-
B
-
7446-09-5
sulfur dioxide

| Conditions | Yield |
|---|---|
| 200°C, 7 days; | A 97% B 97% |
| 100°C, 7 days; | A 19% B 18% |

| Conditions | Yield |
|---|---|
| With benzophenone; chlorine at -80℃; | 92% |

| Conditions | Yield |
|---|---|
| With bromine trifluoride; antimonypentachloride In 1,1,2-Trichloro-1,2,2-trifluoroethane at 20 - 25℃; | 90% |
| With chromium(III) oxyfluoride; hydrogen fluoride at 500℃; |
-
-
79-38-9
Chlorotrifluoroethylene

-
-
53268-50-1
chloro(trifluoromethyl)disulfane

-
A
-
55882-11-6
2,2-dichlorotrifluoroethyl(trifluoromethyl)disulfane

-
B
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

-
C
-
372-07-6
bis(trifluoromethyl)tetrasulfide

-
D
-
55882-10-5
1,2-dichlorotrifluoroethyl(trifluoromethyl)disulfane

| Conditions | Yield |
|---|---|
| Irradiation (UV/VIS); | A n/a B n/a C n/a D 80% |
| Irradiation (UV/VIS); | A n/a B n/a C n/a D 80% |


| Conditions | Yield |
|---|---|
| With iodine at 140 - 165℃; for 4h; | A n/a B 63% |
-
-
79-01-6
Trichloroethylene

-
A
-
354-15-4
1,2,2-trichloro-1,2-difluoroethane

-
B
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

| Conditions | Yield |
|---|---|
| With hydrogen fluoride; fluorine In trichlorofluoromethane at -70℃; for 4h; Product distribution / selectivity; Inert atmosphere; | A 50% B 18% |
-
-
79-38-9
Chlorotrifluoroethylene

-
-
1768-32-7
sulfur bis(trifluoromethyl)amide chloride

-
A
-
34994-73-5
2,4-dichlorohexafluorothiolane

-
B
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

-
C
-
81619-76-3
N,N-bistrifluoromethyl-2,2-dichlorotrifluoroethanesulfenamide

| Conditions | Yield |
|---|---|
| for 528h; Irradiation; Further byproducts given; | A 11% B 16% C 31% D 31% |

-
-
79-38-9
Chlorotrifluoroethylene

-
-
1768-32-7
sulfur bis(trifluoromethyl)amide chloride

-
A
-
423-38-1
1,1,3,4-Tetrachlorohexafluorobutane

-
B
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

-
C
-
81619-76-3
N,N-bistrifluoromethyl-2,2-dichlorotrifluoroethanesulfenamide

| Conditions | Yield |
|---|---|
| for 528h; Irradiation; Further byproducts given; | A 6.5% B 16% C 31% D 31% |

-
-
79-38-9
Chlorotrifluoroethylene

-
-
1768-32-7
sulfur bis(trifluoromethyl)amide chloride

-
A
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

-
B
-
1913-85-5
sulfur bis{bis(trifluoromethyl)amide}

-
C
-
81619-76-3
N,N-bistrifluoromethyl-2,2-dichlorotrifluoroethanesulfenamide

| Conditions | Yield |
|---|---|
| for 528h; Irradiation; Further byproducts given; | A 16% B 15% C 31% D 31% |

-
-
79-38-9
Chlorotrifluoroethylene

-
-
1768-32-7
sulfur bis(trifluoromethyl)amide chloride

-
A
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

-
B
-
81619-76-3
N,N-bistrifluoromethyl-2,2-dichlorotrifluoroethanesulfenamide

| Conditions | Yield |
|---|---|
| for 528h; Irradiation; Further byproducts given; | A 16% B 31% C 31% D 4% |

-
-
79-38-9
Chlorotrifluoroethylene

-
-
1768-32-7
sulfur bis(trifluoromethyl)amide chloride

-
A
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

-
B
-
2714-58-1
disulfur bis{bis(trifluoromethyl)amide}

-
C
-
81619-76-3
N,N-bistrifluoromethyl-2,2-dichlorotrifluoroethanesulfenamide

| Conditions | Yield |
|---|---|
| for 528h; Irradiation; Further byproducts given; | A 11% B 12% C 26% D 26% |

-
-
79-38-9
Chlorotrifluoroethylene

-
-
93460-12-9
α-chlorocarbonylhexafluoroisopropylsulfenyl chloride

-
A
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

-
B
-
83235-80-7
bis-(α-chlorocarbonylhexafluoroisopropyl) disulfide

| Conditions | Yield |
|---|---|
| at 150 - 160℃; for 10h; | A n/a B n/a C 21% |


| Conditions | Yield |
|---|---|
| With hydrogen fluoride; antimonypentachloride at 75 - 100℃; | |
| With antimony (III)-antimony (V)-fluoro chloride ene; hydrogen fluoride; chlorine at 160 - 165℃; unter Druck; | |
| With zirconium(IV) fluoride; hydrogen fluoride at 350 - 360℃; |
-
-
76-11-9
1,1-difluorotetrachloroethane

-
A
-
354-58-5
1,1,1-Trichloro-2,2,2-trifluoroethane

-
B
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

| Conditions | Yield |
|---|---|
| With hydrogen fluoride; pyrographite; lithium fluoride at 375 - 550℃; |
-
-
630-20-6
1,1,1,2-tetrachoroethane

-
A
-
76-14-2
1,2-dichloro-1,1,2,2-tetrafluoroethane

-
B
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

| Conditions | Yield |
|---|---|
| With antimony (III)-antimony (V)-fluoro chloride; hydrogen fluoride; chlorine at 160 - 165℃; unter Druck; | |
| With antimony (III)-antimony (V)-fluoro chloride; hydrogen fluoride; chlorine at 160 - 165℃; unter Druck; | |
| With antimony (III)-antimony (V)-fluoro chloride; hydrogen fluoride; chlorine at 160 - 165℃; unter Druck; |

| Conditions | Yield |
|---|---|
| With hydrogen fluoride; antimonypentachloride at 125℃; |

| Conditions | Yield |
|---|---|
| With nitrogen; copper; fluorine at 70℃; |

| Conditions | Yield |
|---|---|
| With hydrogen fluoride; antimonypentachloride at 125℃; |
-
-
67-72-1
hexachloroethane

-
A
-
76-14-2
1,2-dichloro-1,1,2,2-tetrafluoroethane

-
B
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

| Conditions | Yield |
|---|---|
| With antimony (III)-antimony (V)-fluoro chloride; hydrogen fluoride; chlorine at 160 - 165℃; unter Druck; | |
| With antimony (III)-antimony (V)-fluoro chloride; hydrogen fluoride; chlorine at 160 - 165℃; unter Druck; | |
| With antimony (III)-antimony (V)-fluoro chloride; hydrogen fluoride; chlorine at 160 - 165℃; unter Druck; |

| Conditions | Yield |
|---|---|
| With fluorine at 0℃; | |
| With sulfur tetrafluoride; hydrogen fluoride; chlorine at 20℃; for 10h; Yield given; |
-
-
79-01-6
Trichloroethylene

-
A
-
354-23-4
1,2-dichloro-1,2,2-trifluoroethane

-
B
-
812-04-4
1,1-dichloro-1,2,2-trifluoroethane

-
C
-
354-15-4
1,2,2-trichloro-1,2-difluoroethane

-
D
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

| Conditions | Yield |
|---|---|
| With manganese(III) fluoride at 120℃; | |
| With manganese(III) fluoride at 220℃; |

-
-
79-01-6
Trichloroethylene

-
A
-
354-23-4
1,2-dichloro-1,2,2-trifluoroethane

-
B
-
354-15-4
1,2,2-trichloro-1,2-difluoroethane

-
C
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

-
D
-
76-12-0
CFC-112a

| Conditions | Yield |
|---|---|
| With cobalt (III) fluoride at 120℃; Further byproducts given; |

-
-
79-01-6
Trichloroethylene

-
A
-
75-88-7
1,1,1-trifluoro-2-chloroethane

-
B
-
354-15-4
1,2,2-trichloro-1,2-difluoroethane

-
C
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

-
D
-
76-12-0
CFC-112a

| Conditions | Yield |
|---|---|
| With cobalt (III) fluoride at 120℃; Further byproducts given; |

-
-
79-01-6
Trichloroethylene

-
A
-
812-04-4
1,1-dichloro-1,2,2-trifluoroethane

-
B
-
354-15-4
1,2,2-trichloro-1,2-difluoroethane

-
C
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

-
D
-
76-12-0
CFC-112a

| Conditions | Yield |
|---|---|
| With cobalt (III) fluoride at 120℃; Further byproducts given; |

-
-
79-38-9
Chlorotrifluoroethylene

-
A
-
425-11-6
1,2-Dinitro-1-chlortrifluoraethan

-
B
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

-
C
-
354-27-8
chlorodifluoroacetyl fluoride

-
D
-
354-71-2
1,2-dichloro-1,2,2-trifluoronitrosoethane

| Conditions | Yield |
|---|---|
| With nitrogen(II) oxide; iron(III) chloride Further byproducts given; |

-
-
79-38-9
Chlorotrifluoroethylene

-
A
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

-
B
-
354-61-0
1,2-dichloro-1,2,2-trifluoro-1-iodoethane

-
C
-
661-66-5
1,1-Dichloro-2-iodotrifluoroethane

| Conditions | Yield |
|---|---|
| With Iodine monochloride |

-
-
79-38-9
Chlorotrifluoroethylene

-
A
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

-
B
-
354-27-8
chlorodifluoroacetyl fluoride

-
C
-
354-71-2
1,2-dichloro-1,2,2-trifluoronitrosoethane

| Conditions | Yield |
|---|---|
| With nitrogen(II) oxide; iron(III) chloride |

-
-
79-38-9
Chlorotrifluoroethylene

-
A
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

-
B
-
354-27-8
chlorodifluoroacetyl fluoride

-
C
-
354-71-2
1,2-dichloro-1,2,2-trifluoronitrosoethane

-
D
-
1717-62-0
2-nitro-1,1-dichlorotrifluoroethane

| Conditions | Yield |
|---|---|
| With nitrogen(II) oxide; iron(III) chloride Further byproducts given; |

-
-
79-38-9
Chlorotrifluoroethylene

-
A
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

-
B
-
354-27-8
chlorodifluoroacetyl fluoride

-
C
-
354-71-2
1,2-dichloro-1,2,2-trifluoronitrosoethane

-
D
-
755-62-4
1-chloro-1,2,2-trifluoro-2-nitro-1-nitroso-ethane

| Conditions | Yield |
|---|---|
| With nitrogen(II) oxide; iron(III) chloride Further byproducts given; |


| Conditions | Yield |
|---|---|
| With potassium zinc trihydride at 250 - 320℃; under 7500.75 Torr; for 5h; Temperature; Pressure; Inert atmosphere; Large scale; | 99.22% |
| With ammonium chloride; zinc(II) chloride In methanol; water electrochemical process; | 90% |
| With zinc(II) chloride; zinc In ethanol at 50℃; for 3h; Dehalogenation; | 75% |
-
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

-
-
360-53-2
1,3,3,3-tetrafluoro-1-methoxy-2-trifluoromethyl-propene

| Conditions | Yield |
|---|---|
| at 120℃; for 2.5h; | 98% |
-
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

-
-
109-77-3
malononitrile

-
-
71160-05-9
dimethyl 1,3-propanediimidate dihydrochloride

| Conditions | Yield |
|---|---|
| In hydrogenchloride; methanol | 96% |
-
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

-
A
-
354-23-4
1,2-dichloro-1,2,2-trifluoroethane

-
B
-
79-38-9
Chlorotrifluoroethylene

-
C
-
359-11-5
1,1,2-trifluoroethylene

| Conditions | Yield |
|---|---|
| With hydrogen; silica gel; nickel at 450℃; under 760 Torr; Title compound not separated from byproducts; | A n/a B 95.8% C n/a |
| bismuth(III) chloride; silica gel; palladium at 250℃; Yield given. Yields of byproduct given; | |
| With hydrogen; silica gel; nickel at 450℃; under 760 Torr; Product distribution; other supported Ni catalysts and metal oxides; | |
| bismuth(III) chloride; silica gel; palladium at 250℃; under 760 Torr; Product distribution; other catalysts and modifier; variation of condition: selectivity; |

-
-
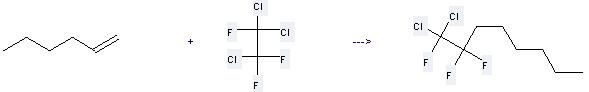
592-76-7
1-Heptene

-
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

-
-
129277-71-0
1,1-Dichloro-1,2,2-trifluoro-nonane

| Conditions | Yield |
|---|---|
| With ammonium peroxydisulfate; sodium formate In N,N-dimethyl-formamide at 40℃; for 4h; | 88.5% |
-
-
592-41-6
1-hexene

-
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

-
-
129277-70-9
1,1-Dichloro-1,2,2-trifluoro-octane

| Conditions | Yield |
|---|---|
| With ammonium peroxydisulfate; sodium formate In N,N-dimethyl-formamide at 40℃; for 3h; other alkenes and alkynes; | 86.5% |
| With ammonium peroxydisulfate; sodium formate In N,N-dimethyl-formamide at 40℃; for 3h; | 86.5% |
-
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

-
-
930-69-8
sodium thiophenolate

-
A
-
91122-75-7
2-chloro-1,1,2-trifluoro-1-(phenyl thio) ethane

-
B
-
91122-73-5
<(2,2-dichloro-1,1,2-trifluoroethyl)thio>benzene

-
C
-
882-33-7
diphenyldisulfane

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide for 0.5h; Product distribution; Mechanism; Ambient temperature; various per(chloro,fluoro)ethanes and conditions; | A 0.9 % Chromat. B 86% C 3 % Chromat. |
| In dimethyl sulfoxide for 0.5h; Ambient temperature; | A 0.9 % Chromat. B 86% C 3 % Chromat. |
| In water; dimethyl sulfoxide for 48h; Ambient temperature; | A 18 % Chromat. B 16 % Chromat. C 38 % Chromat. |


| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 30 - 35℃; for 0.833333h; | 84% |

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 40 - 45℃; for 0.5h; | 81% |

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 45 - 50℃; for 0.5h; | 81% |

| Conditions | Yield |
|---|---|
| With ammonium persulfate; ammonium formate In N,N-dimethyl-formamide at 30 - 40℃; | 80% |
| With ammonium persulfate; ammonium formate In N,N-dimethyl-formamide at 30 - 40℃; Product distribution; Mechanism; also with further polyhalofluoroalkanes; | 80% |
| Irradiation; | |
| With water; sodium formate; titanium(IV) oxide Mechanism; Ambient temperature; Irradiation; |
-
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

-
-
110-83-8
cyclohexene

-
-
129298-65-3
(2,2-Dichloro-1,1,2-trifluoro-ethyl)-cyclohexane

| Conditions | Yield |
|---|---|
| With ammonium peroxydisulfate; sodium formate In N,N-dimethyl-formamide at 50℃; for 8h; | 79.5% |
-
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

-
-
100-67-4
potassium phenolate

-
A
-
456-62-2
1-hydro-1-chloro-2-phenoxyperfluoroethane

-
B
-
95519-55-4
<(2,2-dichloro-1,1,2-trifluoroethyl)oxy>benzene

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide for 1h; Ambient temperature; Yields of byproduct given; | A n/a B 79% |
| In dimethyl sulfoxide for 4h; | A 9% B 37% |

-
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

-
-
591-87-7
Allyl acetate

-
-
129277-72-1
Acetic acid 5,5-dichloro-4,4,5-trifluoro-pentyl ester

| Conditions | Yield |
|---|---|
| With ammonium peroxydisulfate; sodium formate In N,N-dimethyl-formamide at 40℃; for 2h; | 78.4% |
-
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

-
A
-
76-16-4
Hexafluoroethane

-
B
-
76-15-3
Chloropentafluoroethane

-
C
-
76-14-2
1,2-dichloro-1,1,2,2-tetrafluoroethane

| Conditions | Yield |
|---|---|
| With chromium fluoride; magnesium fluoride; hydrogen fluoride at 250 - 500℃; Product distribution; | A 3% B 78% C 19% |


| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 40 - 45℃; for 0.666667h; | 78% |
-
-
5909-26-2
2,4-Bis(methylthio)pyrimidine

-
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

-
-
257957-18-9
4-chloro-2,6-bis(methylthio)pyrimidine

| Conditions | Yield |
|---|---|
| Stage #1: 2,4-Bis(methylthio)pyrimidine With bis(2,2,6,6-tetramethylpiperidin-1-yl)magnesium-bis(lithium chloride) complex In tetrahydrofuran at -20℃; for 1h; Inert atmosphere; Stage #2: 1,1,2-Trichloro-1,2,2-trifluoroethane In tetrahydrofuran at -35 - -20℃; for 3h; Inert atmosphere; regioselective reaction; | 78% |

| Conditions | Yield |
|---|---|
| With bis(fluorosulfuryl) peroxide; antimony pentafluoride; fluorosulphonic acid at 20 - 30℃; for 0.5h; | A 75.4% B n/a |
-
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

-
-
41233-93-6
potassium 2-methylbutan-2-olate

-
B
-
98481-69-7
2-(2,2-Dichloro-1,1,2-trifluoro-ethoxy)-2-methyl-butane

| Conditions | Yield |
|---|---|
| In various solvent(s) for 1h; Ambient temperature; Yields of byproduct given; | A n/a B 75% |


| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 50 - 55℃; for 0.5h; | 75% |

| Conditions | Yield |
|---|---|
| With sodium dithionite; sodium hydrogencarbonate In dimethyl sulfoxide at 60℃; for 6h; | 74% |
| With sodium dithionite; water; sodium hydrogencarbonate In acetonitrile at 40 - 45℃; for 20h; | 56% |
| With sodium dithionite; sodium hydrogencarbonate In acetonitrile at 40 - 45℃; for 20h; | 56% |
-
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

-
-
2215-63-6
1-benzyl-3-hydroxy-1H-indazole

-
-
1300727-50-7
1-benzyl-3-(2,2-dichloro-1,1,2-trifluoroethoxy)-1H-indazole

| Conditions | Yield |
|---|---|
| Stage #1: 1-benzyl-3-hydroxy-1H-indazole With potassium tert-butylate In tert-butyl alcohol at 20℃; for 2h; Stage #2: 1,1,2-Trichloro-1,2,2-trifluoroethane In N,N-dimethyl-formamide at 100℃; for 48h; | 74% |
-
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

-
-
3710-30-3
1,7-Octadiene

-
-
129277-76-5
10,10-Dichloro-9,9,10-trifluoro-dec-1-ene

| Conditions | Yield |
|---|---|
| With ammonium peroxydisulfate; sodium formate In N,N-dimethyl-formamide at 45℃; for 4h; | 72.5% |
-
-
288-13-1
NH-pyrazole

-
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

-
-
909416-36-0
1-(2,2-dichlorotrifluoroethyl)pyrazole

| Conditions | Yield |
|---|---|
| With sodium hydride; tetra-(n-butyl)ammonium iodide In N,N-dimethyl-formamide at 50 - 55℃; for 2h; | 72% |
-
-
681-84-5
tetramethylorthosilicate

-
-
76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

-
-
402-43-7
p-trifluoromethylphenyl bromide

-
-
35692-32-1
trimethoxy(4-(trifluoromethyl)phenyl)silane

| Conditions | Yield |
|---|---|
| With n-butyllithium In diethyl ether; hexane | 72% |

1,1,2-Trichlorotrifluoroethane Consensus Reports
1,1,2-Trichlorotrifluoroethane Standards and Recommendations
ACGIH TLV: TWA 1000 ppm; STEL 1250 ppm; Not Classifiable as a Human Carcinogen
DFG MAK: 500 ppm (3900 mg/m3)
1,1,2-Trichlorotrifluoroethane Analytical Methods
1,1,2-Trichlorotrifluoroethane Specification
The Ethane,1,1,2-trichloro-1,2,2-trifluoro-, with the CAS registry number 76-13-1, is also known as Freon-113. It belongs to the product categories of CFC; Refrigerants; Organics. Its EINECS number is 200-936-1. This chemical's molecular formula is C2Cl3F3 and molecular weight is 187.37. What's more, its systematic name is 1,1,2-trichloro-1,2,2-trifluoroethane. Its classification codes are: (1)Drug / Therapeutic Agent; (2)Reproductive Effect; (3)Skin / Eye Irritant. It is a very unreactive chlorofluorocarbon, that will stay in the atmosphere for a great deal of time if it is released. It was originally used as a coolant in air conditioners and refrigerators, and it was also formerly used as a solvent with which to clean electronics, especially phones. It should be sealed and stored in a cool, ventilated and dry place. Moreover, it should be protected from oxides, heat and fire.
Physical properties of Ethane,1,1,2-trichloro-1,2,2-trifluoro- are: (1)ACD/LogP: 3.20; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 3.19; (4)ACD/LogD (pH 7.4): 3.19; (5)ACD/BCF (pH 5.5): 157.8; (6)ACD/BCF (pH 7.4): 157.8; (7)ACD/KOC (pH 5.5): 1303.23; (8)ACD/KOC (pH 7.4): 1303.23; (9)#H bond acceptors: 0; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 1; (12)Index of Refraction: 1.385; (13)Molar Refractivity: 26.28 cm3; (14)Molar Volume: 112.1 cm3; (15)Polarizability: 10.42×10-24cm3; (16)Surface Tension: 23 dyne/cm; (17)Density: 1.67 g/cm3; (18)Enthalpy of Vaporization: 27.04 kJ/mol; (19)Boiling Point: 50.9 °C at 760 mmHg; (20)Vapour Pressure: 296 mmHg at 25°C.
Preparation: this chemical can be prepared by 1,1,2,2-tetrachloro-1,2-difluoro-ethane at the temperature of 20 - 25 °C. This reaction will need reagents BrF3, SbCl5 and solvent 1,1,2-trichloro-1,2,2-trifluoro-ethane. The yield is about 90%.

Uses of Ethane,1,1,2-trichloro-1,2,2-trifluoro-: it can be used to produce 1,1-dichloro-1,2,2-trifluoro-octane at the temperature of 40 °C. It will need reagents (NH4)2S2O8, HCO2Na·2H2O and solvent dimethylformamide with the reaction time of 3 hours. The yield is about 86.5%.
When you are using this chemical, please be cautious about it as the following:
This chemical is toxic as it has a danger of very serious irreversible effects through inhalation, in contact with skin and if swallowed. It is toxic to aquatic organisms as it may cause long-term adverse effects in the aquatic environment. Moreover, it is dangerous for the ozone layer. When using it, you need wear suitable protective clothing and gloves. You should refer to manufacturer/supplier for information on recovery/recycling. This material and its container must be disposed of as hazardous waste.
You can still convert the following datas into molecular structure:
(1)SMILES: ClC(F)(F)C(Cl)(Cl)F
(2)Std. InChI: InChI=1S/C2Cl3F3/c3-1(4,6)2(5,7)8
(3)Std. InChIKey: AJDIZQLSFPQPEY-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LC50 | inhalation | > 12pph/2H (120000ppm) | Archives des Maladies Professionnelles de Medecine du Travail et de Securite Sociale. Vol. 29, Pg. 381, 1968. | |
| guinea pig | LDLo | oral | > 10gm/kg (10000mg/kg) | National Technical Information Service. Vol. OTS0520705, | |
| mouse | LC50 | inhalation | 260gm/m3/2H (260000mg/m3) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: ATAXIA LUNGS, THORAX, OR RESPIRATION: CYANOSIS | Trudy Leningradskogo Sanitarno-Gigienicheskogo Meditsinskogo Instituta. Vol. 75, Pg. 241, 1963. |
| mouse | LD50 | intravenous | 9gm/kg (9000mg/kg) | AUTONOMIC NERVOUS SYSTEM: OTHER (DIRECT) PARASYMPATHOMIMETIC BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" | National Technical Information Service. Vol. OTS0520355. |
| mouse | LD50 | unreported | 40gm/kg (40000mg/kg) | United States Patent Document. Vol. #4164653. | |
| rabbit | LC50 | inhalation | 59500ppm/2H (59500ppm) | SENSE ORGANS AND SPECIAL SENSES: OTHER: EYE BEHAVIORAL: EXCITEMENT LUNGS, THORAX, OR RESPIRATION: RESPIRATORY STIMULATION | National Technical Information Service. Vol. OTS0520343, |
| rabbit | LD | skin | > 11gm/kg (11000mg/kg) | American Industrial Hygiene Association Journal. Vol. 29, Pg. 521, 1968. | |
| rabbit | LDLo | oral | 17gm/kg (17000mg/kg) | American Industrial Hygiene Association Journal. Vol. 29, Pg. 521, 1968. | |
| rat | LC50 | inhalation | 38500ppm/4H (38500ppm) | BEHAVIORAL: GENERAL ANESTHETIC BEHAVIORAL: EXCITEMENT BEHAVIORAL: ATAXIA | National Technical Information Service. Vol. OTS0520341, |
| rat | LD50 | oral | 43gm/kg (43000mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) GASTROINTESTINAL: OTHER CHANGES SKIN AND APPENDAGES (SKIN): HAIR: OTHER | Journal of Medicinal Chemistry. Vol. 7, Pg. 378, 1964. |
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 76135-82-5
- 761390-58-3
- 76141-89-4
- 76-14-2
- 761435-31-8
- 761439-42-3
- 761440-16-8
- 761440-64-6
- 761440-65-7
- 761440-75-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View