-
Name
o-Phenanthroline
- EINECS 200-629-2
- CAS No. 66-71-7
- Article Data74
- CAS DataBase
- Density 1.25 g/cm3
- Solubility slightly soluble in water
- Melting Point 114-117°C(lit.)
- Formula C12H8N2
- Boiling Point 365.089 °C at 760 mmHg
- Molecular Weight 181.217
- Flash Point 164.756 °C
- Transport Information UN 2811 6.1/PG 3
- Appearance off-white powder
- Safety 45-60-61
- Risk Codes 25-50/53
-
Molecular Structure
-
Hazard Symbols
 T,
T, N
N
- Synonyms 1,10-o-Phenanthroline;4,5-Diazaphenanthrene;β-Phenanthroline;phenanthroline;
- PSA 25.78000
- LogP 2.78300
Synthetic route
-
-
122108-94-5
{(C12H8N2)((C6H5)3P)Cu(O2CC5H7N2)(H2O)}

-
A
-
66-71-7
1,10-Phenanthroline

| Conditions | Yield |
|---|---|
| In acetone byproducts: CO2, H2O, PPh3; N2 bubbled through an acetone suspn. of the Cu-complex (0°C), low ratio Cu complex : acetone; | A n/a B 91% |
| In solid byproducts: CO2, H2O, PPh3; maintained at 120°C under vac. (1E-2 Torr); |

| Conditions | Yield |
|---|---|
| With copper(I) oxide; dmap; N-hydroxyphthalimide; oxygen In acetonitrile at 120℃; for 12h; Sealed tube; | 80% |
| With potassium tert-butylate In o-xylene at 140℃; for 36h; Inert atmosphere; | 15% |
| With platinum; oxygen In methanol at 40℃; under 750.075 Torr; Schlenk technique; Sealed tube; | 100 %Chromat. |
| With tert.-butylhydroperoxide In water at 20℃; for 18h; Sealed tube; | 78 %Spectr. |

-
A
-
66-71-7
1,10-Phenanthroline

-
B
-
14126-11-5
{Ru(1,10-phenanthroline)3}(NO3)2*2H2O

| Conditions | Yield |
|---|---|
| With NaNO3 In methanol byproducts: AgCl; heated to reflux for 15 min; cooled, filtered, concd., addn. of an aq. soln. of NaNO3, pptn. filtered off, washed with diethyl ether, recrystn. (water or water-methanol 9:1); elem. anal.; | A n/a B 75% |


-
-
58034-59-6
{Ag(1,10-phenanthroline)2}ClO4

-
A
-
66-71-7
1,10-Phenanthroline

| Conditions | Yield |
|---|---|
| In methanol byproducts: AgCl; heated to reflux for 15 min; cooled, filtered, concd., addn. of an aq. soln. of NaClO4, pptn. filtered off, washed with diethyl ether, recrystn. (water or water-methanol 9:1); elem. anal.; | A n/a B 75% |

-
-
942267-02-9
(Z)-1,2-di(2-bromopyridin-3-yl)ethene

-
-
66-71-7
1,10-Phenanthroline

| Conditions | Yield |
|---|---|
| With copper In N,N-dimethyl-formamide for 5h; Ullmann coupling; Heating; | 67% |
-
-
65115-91-5
5,6-epoxy-5,6-dihydro-[1,10]phenanthroline

-
-
66-71-7
1,10-Phenanthroline

| Conditions | Yield |
|---|---|
| With 2,4,6-trimereaptotriazine, trisodium salt, nonahydrate In ethanol | 65% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol for 8h; Sealed tube; Reflux; | 64% |

-
A
-
66-71-7
1,10-Phenanthroline

-
B
-
14126-11-5
{Ru(1,10-phenanthroline)3}(NO3)2*2H2O

| Conditions | Yield |
|---|---|
| With NaNO3 In methanol byproducts: AgCl; heated to reflux for 30 min; cooled, filtered, concd., addn. of an aq. soln. of NaNO3, pptn. filtered off, washed with diethyl ether, recrystn. (water or water-methanol 9:1); elem. anal.; | A n/a B 55% |


-
-
58034-59-6
{Ag(1,10-phenanthroline)2}ClO4

-
A
-
66-71-7
1,10-Phenanthroline

| Conditions | Yield |
|---|---|
| In methanol byproducts: AgCl; heated to reflux for 30 min; cooled, filtered, concd., addn. of an aq. soln. of NaClO4, pptn. filtered off, washed with diethyl ether, recrystn. (water or water-methanol 9:1); elem. anal.; | A n/a B 55% |


| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol at 80℃; for 8h; Schlenk technique; | 47% |
-
-
815-68-9
3-acetylpentane-2,4-dione

-
-
158753-17-4
8-amino-7-quinolinecarbaldehyde

-
A
-
66-71-7
1,10-Phenanthroline

-
B
-
3002-77-5
2-methyl-1,10-phenanthroline

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol for 8h; Friedlaender reaction; Heating; | A 33% B 39% |


| Conditions | Yield |
|---|---|
| With sulfuric acid; nitrobenzene | |
| With sulfuric acid; orthoarsenic acid |

| Conditions | Yield |
|---|---|
| With sulfuric acid; orthoarsenic acid; glycerol | |
| With sulfuric acid; orthoarsenic acid; glycerol Reagens 4: HgCl2; |
-
-
23647-26-9
1-methyl-1,10-phenanthrolinium iodide

-
-
66-71-7
1,10-Phenanthroline

| Conditions | Yield |
|---|---|
| With sulfolane; triphenylphosphine at 151℃; Rate constant; |
-
-
2143-61-5
n-Propyl-Radikal

-
A
-
66-71-7
1,10-Phenanthroline

-
B
-
187737-37-7
propene

-
C
-
5331-48-6
n-propylacetamide

-
D
-
80186-77-2
4-propyl-[1,10]phenanthroline

| Conditions | Yield |
|---|---|
| With tris(1,10-phenanthroline)iron(III) tris(hexafluorophosphate) In acetonitrile at 25℃; Rate constant; competition with BrCCL3, other complexes; |

-
-
3531-45-1
n-propyltrimethyltin

-
-
75-05-8
acetonitrile

-
A
-
66-71-7
1,10-Phenanthroline

-
B
-
187737-37-7
propene

-
C
-
5331-48-6
n-propylacetamide

-
D
-
80186-77-2
4-propyl-[1,10]phenanthroline

| Conditions | Yield |
|---|---|
| With sodium hydroxide 1.) 22 deg C; Yield given. Multistep reaction. Yields of byproduct given; |

-
-
3531-46-2
Isopropyl-trimethyl-stannane

-
-
75-05-8
acetonitrile

-
A
-
66-71-7
1,10-Phenanthroline

-
B
-
187737-37-7
propene

-
C
-
1118-69-0
N-isopropylacetamide

-
D
-
80186-78-3
4-isopropyl-1,10-phenanthroline

| Conditions | Yield |
|---|---|
| With sodium hydroxide 1.) 22 deg C; Yield given. Multistep reaction. Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| In water Rate constant; Equilibrium constant; Irradiation; HClO4, H2KPO4, Na2HPO4, Na2B2O7, NaOH, t-BuOH; |


| Conditions | Yield |
|---|---|
| In water Rate constant; Equilibrium constant; Irradiation; HClO4, H2KPO4, Na2HPO4, Na2B2O7, NaOH, t-BuOH; |

-
-
3531-45-1
n-propyltrimethyltin

-
A
-
66-71-7
1,10-Phenanthroline

-
B
-
187737-37-7
propene

-
C
-
5331-48-6
n-propylacetamide

-
D
-
80186-77-2
4-propyl-[1,10]phenanthroline

| Conditions | Yield |
|---|---|
| With sodium hydroxide 1.) acetonitrile, 22 deg C; Yield given. Multistep reaction. Yields of byproduct given; |

-
-
3531-45-1
n-propyltrimethyltin

-
A
-
66-71-7
1,10-Phenanthroline

-
B
-
187737-37-7
propene

-
C
-
5331-48-6
n-propylacetamide

-
D
-
80186-77-2
4-propyl-[1,10]phenanthroline

| Conditions | Yield |
|---|---|
| With sodium hydroxide 1.) acetonitrile, 25 deg C; Yield given. Multistep reaction. Yields of byproduct given; |

-
-
3531-45-1
n-propyltrimethyltin

-
A
-
66-71-7
1,10-Phenanthroline

-
B
-
187737-37-7
propene

-
C
-
5331-48-6
n-propylacetamide

-
D
-
80186-77-2
4-propyl-[1,10]phenanthroline

| Conditions | Yield |
|---|---|
| With sodium hydroxide 1.) acetonitrile, 25 deg C; Yield given. Multistep reaction. Yields of byproduct given; |

-
-
594-27-4
tetramethylstannane

-
A
-
66-71-7
1,10-Phenanthroline

-
B
-
31301-28-7
4-methyl-1,10-phenanthroline

| Conditions | Yield |
|---|---|
| With sodium hydroxide 1.) acetonitrile, 70 deg C, 2 h; Yield given. Multistep reaction; |

-
-
3531-44-0
Ethyltrimethylstannan

-
A
-
80206-19-5
4-ethyl-[1,10]phenanthroline

-
B
-
66-71-7
1,10-Phenanthroline

-
C
-
31301-28-7
4-methyl-1,10-phenanthroline

-
D
-
625-50-3
N-ethylacetamide

| Conditions | Yield |
|---|---|
| With sodium hydroxide 1.) acetonitrile, 22 deg C; Yield given. Multistep reaction. Yields of byproduct given; |

-
-
3531-44-0
Ethyltrimethylstannan

-
-
75-05-8
acetonitrile

-
A
-
80206-19-5
4-ethyl-[1,10]phenanthroline

-
B
-
66-71-7
1,10-Phenanthroline

-
C
-
31301-28-7
4-methyl-1,10-phenanthroline

-
D
-
625-50-3
N-ethylacetamide

| Conditions | Yield |
|---|---|
| With sodium hydroxide 1.) 22 deg C; Yield given. Multistep reaction. Yields of byproduct given; |

-
-
3531-46-2
Isopropyl-trimethyl-stannane

-
A
-
66-71-7
1,10-Phenanthroline

-
B
-
187737-37-7
propene

-
C
-
1118-69-0
N-isopropylacetamide

-
D
-
80186-78-3
4-isopropyl-1,10-phenanthroline

| Conditions | Yield |
|---|---|
| With sodium hydroxide 1.) acetonitrile, 22 deg C; Yield given. Multistep reaction. Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| at 21.85℃; Equilibrium constant; |


| Conditions | Yield |
|---|---|
| In ethanol; dichloromethane |

| Conditions | Yield |
|---|---|
| With sulfuric acid; nitric acid at 160℃; for 3h; | 100% |
| With sulfuric acid; nitric acid at 160℃; | 99% |
| With sulfuric acid; nitric acid at 160℃; for 3h; | 99% |
-
-
66-71-7
1,10-Phenanthroline

-
-
27318-90-7
1,10-phenanthroline-5,6-dione

| Conditions | Yield |
|---|---|
| With sulfuric acid; nitric acid; sodium bromide at 90℃; for 2h; | 100% |
| With sulfuric acid; nitric acid; potassium bromide at 130℃; for 3h; | 98.1% |
| With sulfuric acid; nitric acid; potassium bromide at 95℃; for 3h; Cooling with ice; Sealed tube; Schlenk technique; | 97% |
-
-
66-71-7
1,10-Phenanthroline

-
-
109-64-8
1,3-dibromo-propane

-
-
15302-99-5
6,7-dihydro-5H-[1,4]diazepino[1,2,3,4-l,m,n][1,10]phenanthroline-4,8-diium dibromide

| Conditions | Yield |
|---|---|
| In chlorobenzene at 70 - 120℃; | 100% |
| In nitrobenzene at 120℃; | 100% |
| for 12h; Reflux; | 99% |
-
-
66-71-7
1,10-Phenanthroline

-
-
12146-37-1, 124717-04-0
(bicyclo[2.2.1]hepta-2,5-diene)tetracarbonylmolybdenum(0)

-
A
-
15740-78-0
(1,10-phenanthroline)molybdenum tetracarbonyl

-
B
-
121-46-0
bicyclo[2.2.1]hepta-2,5-diene

| Conditions | Yield |
|---|---|
| In tetrahydrofuran reaction in a calorimeter under argon; | A 100% B n/a |

-
-
7647-01-0
hydrogenchloride

-
-
66-71-7
1,10-Phenanthroline

-
-
10466-65-6
potassium perrhenate

-
B
-
17428-51-2
o-phenanthrolinium oxopentachlororhenate(V)

| Conditions | Yield |
|---|---|
| With hydroquinone In acetic anhydride; acetic acid under N2 at room temp.; passing HCl gas through soln. of KReO4 in acetic acid and (Ac)2O till clear soln. (rhenate), mixed with hydroquinone inacetic acid, pptn. of KReOCl5, to mother liquor addn. of phenanthrolinen AcOH contg. (Ac)2O, pptn.; filtered, washed (acetic acid), kept in vacuo for 5 h; elem. anal.; | A 100% B 100% |

-
-
66-71-7
1,10-Phenanthroline

-
-
10466-65-6
potassium perrhenate

-
-
17428-51-2
o-phenanthrolinium oxopentachlororhenate(V)

| Conditions | Yield |
|---|---|
| With hydroquinone In hydrogenchloride under N2 at room temp.; KReO4 suspended in concd. HCl, shaken, addn. ofhydroquinone in concd. HCl, yellow soln. obtained, addn. of phenanthroline, pptn.; filtered, washed (acetic acid), kept in vacuo for 5 h; | 100% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran (Ar); stirring (5 h); washing (hexane), drying (vac.); elem. anal.; | 100% |

-
-
66-71-7
1,10-Phenanthroline

-
-
52462-29-0
[ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2

-
-
861392-78-1
chloro(p-cymene)(η2-1,10-phenanthroline-κ2N)ruthenium

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 3h; Inert atmosphere; Schlenk technique; | 100% |
| In dichloromethane inert atm.; 2 equiv of phenanthroline was added to a suspn. of complex in CH2Cl2, the mixt. was stirred for 3 h at room temp.; evapd. to dryness, dissolved in water, filtered, evapd. to dryness; elem. anal.; | 99% |
| In dichloromethane at 20℃; for 0.5h; | 51% |
-
-
66-71-7
1,10-Phenanthroline

| Conditions | Yield |
|---|---|
| In toluene Ar atm.; stirring; concn., addn. of hexane, crystn. (-30°C, overnight), filtn., drying (vac., 40°C); | 100% |
-
-
66-71-7
1,10-Phenanthroline

-
-
64443-05-6
tetrakis(actonitrile)copper(I) hexafluorophosphate

-
-
124318-69-0
2,9-bis(2,6-dimethoxyphenyl)-3-ethynyl-[1,10]-phenanthroline

-
-
1196061-73-0
Cu(C12H6N2(C6H3(OCH3)2)2)(C12H8N2)(1+)*PF6(1-)=CuC40H32N4O4PF6

| Conditions | Yield |
|---|---|
| In [D3]acetonitrile | 100% |

-
-
66-71-7
1,10-Phenanthroline

-
-
7783-68-8
niobium pentafluoride

-
-
4648-54-8
trimethylsilylazide

-
-
1628265-33-7
[Nb(N3)4(1,10-phen)2][Nb(N3)6]

| Conditions | Yield |
|---|---|
| In acetonitrile at -196 - 20℃; for 6h; | 100% |

-
-
66-71-7
1,10-Phenanthroline

-
-
7783-71-3
tantalum pentafluoride

-
-
4648-54-8
trimethylsilylazide

-
-
1628265-35-9
[Ta(N3)4(1,10-phen)2][Ta(N3)6]

| Conditions | Yield |
|---|---|
| In acetonitrile at -196 - 20℃; for 6h; | 100% |

-
-
66-71-7
1,10-Phenanthroline

-
-
64443-05-6
tetrakis(actonitrile)copper(I) hexafluorophosphate

| Conditions | Yield |
|---|---|
| In dichloromethane-d2 | 100% |

-
-
66-71-7
1,10-Phenanthroline

-
-
1613741-33-5
2-(6-(2,4,6-trimethylphenyl)pyridin-2-yl)-9-(2,4,6-trimethylphenyl)[1,10]phenanthroline

-
-
54010-75-2
zinc trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| In dichloromethane-d2; [D3]acetonitrile | 100% |

-
-
66-71-7
1,10-Phenanthroline

-
-
1613741-33-5
2-(6-(2,4,6-trimethylphenyl)pyridin-2-yl)-9-(2,4,6-trimethylphenyl)[1,10]phenanthroline

| Conditions | Yield |
|---|---|
| In dichloromethane-d2; [D3]acetonitrile | 100% |

-
-
108-86-1
bromobenzene

-
-
66-71-7
1,10-Phenanthroline

-
-
15302-81-5
6,7-dihydro-5H-[1,4]diazepino[1,2,3,4-lmn][1,10]phenanthroline-4,8-diium

| Conditions | Yield |
|---|---|
| at 115℃; for 4h; | 100% |
-
-
66-71-7
1,10-Phenanthroline

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 30h; Inert atmosphere; Schlenk technique; | 100% |
-
-
66-71-7
1,10-Phenanthroline

| Conditions | Yield |
|---|---|
| In dichloromethane for 30h; Inert atmosphere; Schlenk technique; | 100% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 1h; | 100% |
-
-
66-71-7
1,10-Phenanthroline

-
-
718-64-9
1,1,1,3,3,3-hexafluoro-2-phenylisopropyl alcohol

-
-
1197008-50-6
C30H37N3W

| Conditions | Yield |
|---|---|
| Stage #1: 1,1,1,3,3,3-hexafluoro-2-phenylisopropyl alcohol; C30H37N3W In benzene at 20℃; for 0.5h; Stage #2: 1,10-Phenanthroline In benzene at 20℃; for 1h; | 100% |

-
-
66-71-7
1,10-Phenanthroline

| Conditions | Yield |
|---|---|
| In water; N,N-dimethyl-formamide for 0.0833333h; | 100% |


| Conditions | Yield |
|---|---|
| In acetonitrile at -196 - 20℃; for 16h; | 100% |

-
-
66-71-7
1,10-Phenanthroline

-
-
888020-56-2
tris(p-trifluoromethylphenyl)phosphine gold bis(trifluoromethanesulfonyl)imidate

| Conditions | Yield |
|---|---|
| In dichloromethane Inert atmosphere; | 100% |
-
-
66-71-7
1,10-Phenanthroline

| Conditions | Yield |
|---|---|
| In dichloromethane Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| In dichloromethane Inert atmosphere; | 100% |
-
-
66-71-7
1,10-Phenanthroline

-
-
1160111-28-3
[(Cy3P)Au]N(trifluoromethylsulfonyl)2

| Conditions | Yield |
|---|---|
| In dichloromethane Inert atmosphere; | 100% |
-
-
66-71-7
1,10-Phenanthroline

-
-
1644306-41-1
(p-tolyl)3PAuN(trifluoromethanesulfonyl)2

| Conditions | Yield |
|---|---|
| In dichloromethane Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| In dichloromethane Inert atmosphere; | 100% |
-
-
66-71-7
1,10-Phenanthroline

-
-
866395-16-6, 1246810-76-3
[bis(trifluoromethanesulfonyl)imidate](triphenylphosphine)gold(I)

| Conditions | Yield |
|---|---|
| In dichloromethane Inert atmosphere; | 100% |
-
-
66-71-7
1,10-Phenanthroline

-
A
-
85152-70-1
tris(1,10-phenanthroline)iron(II) triflate

| Conditions | Yield |
|---|---|
| In [D3]acetonitrile | A 100% B n/a |

1,10-Phenanthroline Consensus Reports
1,10-Phenanthroline Specification
The 1,10-Phenanthroline, with the CAS registry number 66-71-7 and EINECS registry number 200-629-2, has the systematic name of 1,10-o-Phenanthroline. And the molecular formula of this chemical is C12H8N2. It is a kind of off-white powder, and belongs to the following product categories: Industrial/Fine Chemicals; Heterocyclic Building Blocks; N-Containing.
The physical properties of 1,10-Phenanthroline are as following: (1)ACD/LogP: 2.25; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.085; (4)ACD/LogD (pH 7.4): 2.244; (5)ACD/BCF (pH 5.5): 20.669; (6)ACD/BCF (pH 7.4): 29.861; (7)ACD/KOC (pH 5.5): 273.539; (8)ACD/KOC (pH 7.4): 395.176; (9)#H bond acceptors: 2; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 0; (12)Polar Surface Area: 25.78 Å2; (13)Index of Refraction: 1.74; (14)Molar Refractivity: 58.12 cm3; (15)Molar Volume: 144.113 cm3; (16)Polarizability: 23.041×10-24cm3; (17)Surface Tension: 61.295 dyne/cm; (18)Density: 1.25 g/cm3; (19)Flash Point: 164.756 °C; (20)Enthalpy of Vaporization: 58.73 kJ/mol; (21)Boiling Point: 365.089 °C at 760 mmHg; (22)Vapour Pressure: 0 mmHg at 25°C.
Preparation and uses of 1,10-Phenanthroline: It can be prepared by heating the o-phenylenediamine and glycerol, nitrobenzene and sulfuric acid. And it is often used as electroplating additives and analytical reagent. It is also used as redox indicator. What's more, it is used as reagent for the determination of iron, palladium, vanadium, copper and iron.
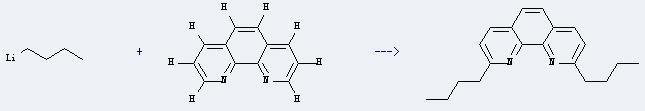
Chemical reaction: It is used in the synthesis of other chemicals. For example, it can react with butyllithium to produce 2,9-di-n-butyl-1,10-phenanthroline. This reaction will need reagent H2O, and the solvents MnO2 and toluene. The reaction time is 16 hours with temperature of 20°C, and the yield is about 62%.
You should be cautious while dealing with this chemical. It is toxic if swallowed. It is also very toxic to aquatic organisms, and may cause long-term adverse effects in the aquatic environment . Therefore, you had better take the following instructions: This material and/or its container must be disposed of as hazardous waste; Avoid release to the environment. Refer to special instructions safety data sheet; In case of accident or if you feel unwell, seek medical advice immediately (show label where possible).
You can still convert the following datas into molecular structure:
(1)SMILES: c1cc2ccc3cccnc3c2nc1
(2)InChI: InChI=1/C12H8N2/c1-3-9-5-6-10-4-2-8-14-12(10)11(9)13-7-1/h1-8H
(3)InChIKey: DGEZNRSVGBDHLK-UHFFFAOYAW
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 75mg/kg (75mg/kg) | KIDNEY, URETER, AND BLADDER: OTHER CHANGES IN URINE COMPOSITION KIDNEY, URETER, AND BLADDER: OTHER CHANGES | Journal of Pharmacology and Experimental Therapeutics. Vol. 196, Pg. 478, 1976. |
| mouse | LD50 | intravenous | 18mg/kg (18mg/kg) | Journal of Pharmacology and Experimental Therapeutics. Vol. 137, Pg. 1, 1962. |
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 66719-32-2
- 66719-33-3
- 66720-17-0
- 66722-44-9
- 66722-57-4
- 6672-30-6
- 66723-45-3
- 667-27-6
- 66-72-8
- 66728-98-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View