-
Name
1,2-Butadiene
- EINECS 209-674-2
- CAS No. 590-19-2
- Article Data92
- CAS DataBase
- Density 0.612 g/cm3
- Solubility Soluble in ethanol, ether and benzene, insoluble in water
- Melting Point -136.2oC
- Formula C4H6
- Boiling Point 10.7 °C at 760 mmHg
- Molecular Weight 54.0916
- Flash Point
- Transport Information UN 2037 2.1
- Appearance colorless gas
- Safety 16-23-24/25
- Risk Codes 12
-
Molecular Structure
-
Hazard Symbols
 F+
F+
- Synonyms 1-Methylallene;Allene, methyl-;Butadiene-1,2;Methylallene;
- PSA 0.00000
- LogP 1.34740
Synthetic route
-
-
41998-65-6
3-methylpent-4-yn-2-ol

-
-
590-19-2
2,3-dimethylbutene

| Conditions | Yield |
|---|---|
| at 660℃; under 0.001 Torr; | 98% |
-
-
3100-04-7
Methylcyclopropen

-
A
-
503-17-3
dimethylacetylene

-
B
-
590-19-2
2,3-dimethylbutene

-
C
-
106-99-0
buta-1,3-diene

| Conditions | Yield |
|---|---|
| With sulphur hexafluoride at 230.2℃; under 20.5 Torr; for 1.5h; Product distribution; Rate constant; Kinetics; var. time, var. pressure; | A 91.31% B 1.84% C 6.85% |
| Product distribution; Quantum yield; Irradiation; | |
| In gas under 0.1 - 6 Torr; Mechanism; Product distribution; Irradiation; |

-
-
21020-24-6
3-chloro-but-1-yne

-
-
590-19-2
2,3-dimethylbutene

| Conditions | Yield |
|---|---|
| 61% |

| Conditions | Yield |
|---|---|
| at 20℃; under 50 Torr; Kinetics; radical cross-combination reaction; Photolysis; | A 60% B 40% |


| Conditions | Yield |
|---|---|
| With iron at 23 - 110℃; under 29420.3 Torr; Hydrogenation.in Gegenwart eines Polymerisationsinhibitors; |

| Conditions | Yield |
|---|---|
| With cefaloridine at 280℃; | |
| Cs-exchanged H(x)Cs30Na19(AlO2)54(SiO2)138 zeolite Ambient temperature; |

| Conditions | Yield |
|---|---|
| at 275℃; Leiten ueber Floridin; |

| Conditions | Yield |
|---|---|
| at 280℃; |


| Conditions | Yield |
|---|---|
| With cefaloridine at 280℃; |
-
-
513-35-9
2-methyl-but-2-ene

-
A
-
187737-37-7
propene

-
B
-
74-85-1
ethene

-
C
-
590-19-2
2,3-dimethylbutene

-
D
-
78-79-5
isoprene

| Conditions | Yield |
|---|---|
| at 750℃; under 15 Torr; |

-
-
513-35-9
2-methyl-but-2-ene

-
A
-
91-20-3
naphthalene

-
B
-
74-85-1
ethene

-
C
-
590-19-2
2,3-dimethylbutene

-
D
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| at 750℃; Produkt 5:Anthracen; |


| Conditions | Yield |
|---|---|
| at 1300 - 1500℃; Pyrolysis; |
-
-
2465-56-7
methylene

-
-
142-96-1
dibutyl ether

-
A
-
628-28-4
butyl methyl ether

-
B
-
590-19-2
2,3-dimethylbutene

-
C
-
18636-66-3
n-butyl n-pentyl ether

-
-
52111-97-4
2,3-dibromo-but-1-ene

-
-
590-19-2
2,3-dimethylbutene

| Conditions | Yield |
|---|---|
| With ethanol; zinc |

| Conditions | Yield |
|---|---|
| at 500℃; Pyrolysis; |

| Conditions | Yield |
|---|---|
| Mechanism; |

| Conditions | Yield |
|---|---|
| at 600℃; |

-
-
74-98-6
propane

-
A
-
187737-37-7
propene

-
B
-
34557-54-5
methane

-
C
-
74-85-1
ethene

-
D
-
590-19-2
2,3-dimethylbutene

| Conditions | Yield |
|---|---|
| at 700 - 1000℃; bei der termischen Zersetzung; Produkt 5: Benzol; Produkt 6: Acetylen; Produkt 7: Kohlenstoff; Produkt 8: Wasserstoff; |

-
-
79630-70-9
1,2,2,3-tetrachloro-butane

-
-
590-19-2
2,3-dimethylbutene

| Conditions | Yield |
|---|---|
| With zinc copper; ethanol |
-
-
20629-39-4, 20629-62-3, 79629-38-2
1,2-dibromo-2-butene

-
-
590-19-2
2,3-dimethylbutene

| Conditions | Yield |
|---|---|
| With ethanol; zinc |

| Conditions | Yield |
|---|---|
| With aluminum oxide at 360 - 460℃; |

| Conditions | Yield |
|---|---|
| nach dem Houdry-Prozess; |

| Conditions | Yield |
|---|---|
| at 600℃; |

| Conditions | Yield |
|---|---|
| at 800℃; under 147.1 Torr; bei Verduennung mit Stickstoff und Kontaktzeit von 0.07 sec; |

| Conditions | Yield |
|---|---|
| at 760 - 833℃; bei Verduennung mit Wasserdampf und einer Kontaktzeit von 0.005-0.1 sec; |

| Conditions | Yield |
|---|---|
| at 650℃; bei Verduennung mit Wasserdampf und Kontaktzeit von 0.4-1 sec; |

-
-
590-19-2
2,3-dimethylbutene

| Conditions | Yield |
|---|---|
| at 750℃; under 0.001 Torr; |

| Conditions | Yield |
|---|---|
| tetrahydrofuran; samarium at 79.9℃; |
-
-
107-00-6
but-1-yne

-
A
-
503-17-3
dimethylacetylene

-
B
-
590-19-2
2,3-dimethylbutene

-
C
-
106-99-0
buta-1,3-diene

| Conditions | Yield |
|---|---|
| calcium oxide at 30℃; Product distribution; |

-
-
503-17-3
dimethylacetylene

-
A
-
106-98-9
1-butylene

-
B
-
590-18-1
(Z)-2-Butene

-
C
-
590-19-2
2,3-dimethylbutene

| Conditions | Yield |
|---|---|
| With hydrogen; tetrahydrofuran; samarium variation of rare earth catalyst, selectivity; | |
| With hydrogen; tetrahydrofuran; samarium at 79.9℃; |

-
-
590-19-2
2,3-dimethylbutene

-
-
200010-86-2
2-(methyldiphenylsilyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane

| Conditions | Yield |
|---|---|
| With catalyst:Pd(dba)2/R-1,1'-bina-2yl-P(3,5C6H3-Me2)2 In toluene 0°C, 22-90 h, bis(dibenzylideneacetone) palladium / (R)-(1,1')binaphthalenyl-2-yl-bis-(3,5-dimethyl-phenyl)-phosphane as catalyst; | 96% |
-
-
590-19-2
2,3-dimethylbutene

| Conditions | Yield |
|---|---|
| In dichloromethane (Ar, Schlenk) methylallene was passed through a stirred soln. of complexin CH2Cl2 for 1 min at a rate 0.1 mL/s, stirred under methylallene atm. at ambient temp. for 24 h; reduced in vol., diethyl ether was added, ppt. was washed with diethyl ether (twice) and dried in vac.; elem. anal.; | 94% |
-
-
590-19-2
2,3-dimethylbutene

-
-
612835-98-0
(1R,2R,6S,8R)-4-(Dimethyl-phenyl-silanyl)-2,9,9-trimethyl-3,5-dioxa-4-bora-tricyclo[6.1.1.02,6]decane

| Conditions | Yield |
|---|---|
| With (C5H5)(allyl)Pd; (R)-Ph2PC10H6C10H7 In toluene allene (1.2 equiv.) added to mixt. of P ligand and Pd compd. in toluene at room temp.; (R)-silylborane added; mixt. stirred at room temp. for 8-24 h; bulb-to-bulb distd. under vac.; ratio of (R,S) to (R,R) = 93:7; | 92% |

-
-
590-19-2
2,3-dimethylbutene

| Conditions | Yield |
|---|---|
| With HBF4*Et2O In chlorobenzene under inert gas; addn. of HBF4*OEt2 to a soln of (C5H5)Re(NO)(PPh3)(CH3) at -45°C with stirring, after 30 min addn. of 1,2-butadiene, stirring at room temp. for 5 d;; evapn., pptn., filtration, dissolving (CH2Cl2), addn. to hexane, pptn., crystn. (CH2Cl2/ether); elem. anal.; | 90% |

| Conditions | Yield |
|---|---|
| With [2,2]bipyridinyl; carbon dioxide In tetrahydrofuran MeCH=C=CH2 was added at -78°C to a suspn. of (COD)2Ni and bipy in THF, reaction vessel was evacuated, CO2 admitted at -78°C, mixt. was warmed at 20°C and stirred for 48 h; soln. was concd. in vacuo, pentane added, ppt. was filtered off, washedwith pentane and dried in vacuo; elem. anal.; | 88.5% |
-
-
590-19-2
2,3-dimethylbutene

-
-
10124-62-6
1,2-dimethoxy-1,1,2,2-tetramethyldisilane

| Conditions | Yield |
|---|---|
| With butene-2; tetrakis(triphenylphosphine) palladium(0) at 120℃; for 15h; | 88% |
| With tetrakis(triphenylphosphine) palladium(0) In benzene at 120℃; for 15h; | 88% |
-
-
590-19-2
2,3-dimethylbutene

-
-
185990-03-8
dimethylphenyl(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)silane

| Conditions | Yield |
|---|---|
| With ethriol phosphite; tris(dibenzylideneacetone)dipalladium (0) In tetrahydrofuran at 80℃; for 9h; Addition; | 88% |
| With catalyst: Pd2(dba)3 + etpo In tetrahydrofuran heating catalyst at 80°C for 5 min, addn. of diene and B-compound, heating at 80°C for 9 h; TLC; | 88% |

| Conditions | Yield |
|---|---|
| Stage #1: 2,3-dimethylbutene; acetophenone With (R)-Cl-MeO-BiPhep; bis(pinacol)diborane; copper(l) chloride; sodium t-butanolate In tetrahydrofuran at 22℃; for 18h; Inert atmosphere; Stage #2: With sodium perborate In tetrahydrofuran; water at 22℃; for 1h; Inert atmosphere; enantioselective reaction; | A n/a B 88% |

-
-
590-19-2
2,3-dimethylbutene

-
-
18107-29-4
methoxypentamethyldisilane

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0) In benzene at 120℃; for 15h; | 87% |

| Conditions | Yield |
|---|---|
| Stage #1: 2,3-dimethylbutene; acetophenone With 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; bis(pinacol)diborane; copper(l) chloride; sodium t-butanolate In tetrahydrofuran at 22℃; for 18h; Inert atmosphere; Stage #2: With sodium perborate In tetrahydrofuran; water at 22℃; for 1h; Inert atmosphere; diastereoselective reaction; | 87% |
-
-
590-19-2
2,3-dimethylbutene

-
-
18107-32-9
1,1,2,2-tetramethoxy-1,2-dimethyl-disilane

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0) In benzene at 120℃; for 15h; | 86% |

-
-
590-19-2
2,3-dimethylbutene

| Conditions | Yield |
|---|---|
| With trimethylamine oxide In dichloromethane byproducts: CO; (Ar); passing methylallene through a soln. of cluster in CH2Cl2, addn. of amine oxide, stirring for 30 min; filtration, addn. of pentane, washing ppt. with ether, drying in argon; elem. anal.; | 86% |
-
-
590-19-2
2,3-dimethylbutene

-
-
1380397-20-5
[IrOs(CO)3(μ-η3:κ1-CH2CCHCH3)(μ-Ph2PCH2PPh2)2][BF4]

| Conditions | Yield |
|---|---|
| In dichloromethane byproducts: CO; (Ar); CH3CHCCH2 passed through a soln. of Ir complex for 1 min, stirred under CH3CHCCH2 for 4 h at ambient temp.; concd., pptd. (Et2O), washed (Et2O), dried (vac.); elem. anal.; | 85% |
| With (CH3)3NO In not given (Ar); |
-
-
590-19-2
2,3-dimethylbutene

-
-
13528-88-6
1,1,2-trichloro-1,2,2-trimethyldisilane

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0) In 1,3,5-trimethyl-benzene at 100℃; for 5h; | 84% |

| Conditions | Yield |
|---|---|
| With butene-2; tetrakis(triphenylphosphine) palladium(0) at 100℃; for 5h; | 84% |
-
-
590-19-2
2,3-dimethylbutene

-
A
-
59409-51-7
3-methyl-undeca-1,2-diene

-
B
-
56956-47-9
2,3-dodecadiene

-
C
-
77764-66-0
3-methylundec-1-yne

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran; hexane -78 deg C, 30 min -> room temperature; | A 15% B 84% C 1% |


| Conditions | Yield |
|---|---|
| With isopropyl alcohol; caesium carbonate In ethyl acetate; 1,2-dichloro-ethane at 75℃; for 14h; | 83% |

-
-
590-19-2
2,3-dimethylbutene

| Conditions | Yield |
|---|---|
| In not given in react. of (HB(pz)3)(1-) with the solvated cation (Pt(CH3)(COD))(1+) an insol. and probably polymeric product is formed; this reacts readily with substituted allene;; | 83% |
| In not given in react. of (HB(pz)3)(1-) with the solvated cation (Pt(CH3)(COD))(1+) an insol. and probably polymeric product is formed; this reacts readily with substituted allene;; | 83% |


| Conditions | Yield |
|---|---|
| caesium carbonate In ethyl acetate; 1,2-dichloro-ethane at 75℃; for 14h; | 82% |

| Conditions | Yield |
|---|---|
| Stage #1: 2,3-dimethylbutene; cyclohexane carbonitrile; bis(pinacol)diborane With (2R)-1-[(1R)-1-[bis(1,1-dimethylethyl)phosphino]ethyl]-2-(diphenylphosphino)ferrocene; mesitylcopper(I) In methanol; diethyl ether at -40℃; for 10h; Stage #2: With aluminium(III) triflate In methanol for 2h; Stage #3: With methanol; lithium borohydride at -78℃; for 4h; stereoselective reaction; | 81% |

-
-
548757-70-6, 33154-75-5, 12126-69-1, 548757-71-7, 39330-67-1
[Ni(1,5,9-cyclododecatriene)]

-
-
590-19-2
2,3-dimethylbutene

-
-
23743-26-2
1,2-bis-(dicyclohexylphosphino)ethane

| Conditions | Yield |
|---|---|
| With carbon dioxide In tetrahydrofuran MeCH=C=CH2 was added at -78°C to a suspn. of (CDT)Ni and diphosphine in THF, reaction vessel was evacuated, CO2 admitted at -78°C, mixt. was warmed at 20°C and stirred for 48 h; soln. was concd. in vacuo, pentane added, ppt. was filtered off, washedwith pentane and dried in vacuo; elem. anal.; | 80.2% |


| Conditions | Yield |
|---|---|
| With <2,6-(ipr)2-C6H3-N=CH-CH=N-2,6-(ipr)2C6H3>Pd-(COOMe)4-cyclopentadiene In toluene at 40℃; for 48h; | 80% |
-
-
590-19-2
2,3-dimethylbutene

-
-
323185-93-9
[IrRu(CO)4(bis(diphenylphosphino)methane)2][BF4]

| Conditions | Yield |
|---|---|
| In dichloromethane (N2); Schlenk technique; stirred soln. of Ir-Ru complex in CH2Cl2 was satd. with methylallene; stirred at ambient temp. for 24 h; pentane added; filtered; rinsed (pentane); dried (vac.); | 80% |

| Conditions | Yield |
|---|---|
| With isopropyl alcohol; caesium carbonate In ethyl acetate; 1,2-dichloro-ethane at 75℃; for 14h; | 79% |
-
-
590-19-2
2,3-dimethylbutene

-
-
13089-11-7
methyl 3,3,3-trifluoropyruvate

| Conditions | Yield |
|---|---|
| at 100℃; for 24h; | 78% |
-
-
590-19-2
2,3-dimethylbutene

-
-
107836-65-7
1-phenylethan-1-iminium chloride

-
-
73183-34-3
bis(pinacol)diborane

| Conditions | Yield |
|---|---|
| With C57H66N2O3S; copper(l) chloride; sodium t-butanolate In tetrahydrofuran at 22℃; for 24h; stereoselective reaction; | 78% |


| Conditions | Yield |
|---|---|
| With isopropyl alcohol; caesium carbonate In ethyl acetate; 1,2-dichloro-ethane at 75℃; for 14h; | 77% |
-
-
590-19-2
2,3-dimethylbutene

-
-
73183-34-3
bis(pinacol)diborane

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0) In toluene room temp., 14 h; chromy. on silica; | 77% |
| With tris-(dibenzylideneacetone)dipalladium(0) In toluene room temp., 14 h; chromy. on silica; | 68% |
1,2-Butadiene Specification
1,2-Butadiene is a colourless flammable gas at room temperature and atmospheric pressure with the formula C4H6. The IUPAC name of this chemical is buta-1,2-diene. It is soluble in ethanol, ether and benzene, insoluble in water.
Preparation: 1,2-Butadiene can be obtained by many methods. It can be extracted from a mixed butadiene stream using a distillation process. It also can be obtained by 3-methyl-pent-4-yn-2-ol. This reaction reacts at temperature of 66 °C and pressure of 0.001. The yield is 98%.

Uses: 1,2-Butadiene is mainly used as feedstock in the manufacturing of synthetic elastomers. It probably has some anesthetic activity and can act as a simple asphyxiant. Moreover, it is also used in organic synthesis. For example: it can react with hexamethyl-disilane to get 2,3-bis-trimethylsilanyl-but-1-ene. This reaction needs reagent but-2-ene and catalytic agent Pd(PPh3)4 at temperature of 120 °C. The reaction time is 15 hours. The yield is 73%.
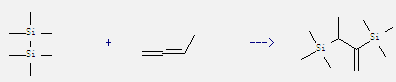
Safty: 1,2-Butadiene is extremely flammable, so peole should keep it away from sources of ignition. As it is a chemical, people must not breathe vapour and avoid contact with skin and eyes. The product must be stored in air bottle in a cool place.
Other properties: (1)ACD/LogP: 1.97; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.97; (4)ACD/LogD (pH 7.4): 1.97; (5)ACD/BCF (pH 5.5): 18.6; (6)ACD/BCF (pH 7.4): 18.6; (7)ACD/KOC (pH 5.5): 282.07; (8)ACD/KOC (pH 7.4): 282.07; (9)#H bond acceptors: 0; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 0; (12)Polar Surface Area: 0 Å2; (13)Index of Refraction: 1.376; (14)Molar Refractivity: 20.28 cm3; (15)Molar Volume: 88.3 cm3; (16)Polarizability: 8.04×10-24 cm3; (17)Surface Tension: 6 dyne/cm; (18)Density: 0.612 g/cm3; (19)Flash Point: °C; (20)Enthalpy of Vaporization: 24.02 kJ/mol; (21)Boiling Point: 10.7 °C at 760 mmHg; (22)Vapour Pressure: 1260 mmHg at 25°C.
Structure Descriptors:
1. Smiles:C(=C=C)C
2. InChI:InChI=1/C4H6/c1-3-4-2/h4H,1H2,2H3
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 59020-09-6
- 59020-10-9
- 59020-44-9
- 59020-85-8
- 59020-90-5
- 59021-02-2
- 59022-46-7
- 59025-03-5
- 5902-51-2
- 59025-55-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View