-
Name
1,3-Bis(aminomethyl)benzene
- EINECS 216-032-5
- CAS No. 1477-55-0
- Article Data62
- CAS DataBase
- Density 1.052 g/cm3
- Solubility Miscible
- Melting Point 14 °C
- Formula C8H12N2
- Boiling Point 247 °C at 760 mmHg
- Molecular Weight 136.197
- Flash Point 130.4 °C
- Transport Information
- Appearance clear slightly yellow liquid
- Safety 26-36/37/39-45-61-28A
- Risk Codes 21/22-23-34-43-52/53
-
Molecular Structure
-
Hazard Symbols
 T,
T, C
C
- Synonyms a,a'-Diamino-m-xylene;meta-Xylylenediamine( MXDA );m-Xylene-a,a'-diamine (6CI,8CI);1,3-Bis(aminomethyl)benzene;1,3-Xylylenediamine;3-(Aminomethyl)benzylamine;Epilink MX;Euredur 22;NSC 61568;Shoamine X;m-(a,a'-Diamino)xylene;m-Xylylenediamine;
- PSA 52.04000
- LogP 2.00460
Synthetic route
-
-
24964-64-5
3-Cyanobenzaldehyde

-
-
1477-55-0
1,3-di(aminomethyl)benzene

| Conditions | Yield |
|---|---|
| With ammonia; hydrogen In methanol at 100℃; under 37503.8 Torr; for 5h; | 100% |
| With ammonia; hydrogen In methanol at 80℃; under 22502.3 Torr; for 5h; | 74% |

| Conditions | Yield |
|---|---|
| With ammonia; hydrogen In methanol at 100℃; under 22502.3 Torr; for 2h; Pressure; Reagent/catalyst; Temperature; | 99% |
| With hydrogen; cobalt/diatomaceous earth catalyst "G67", cobalt content=56percent; pretreatment is given in full text In 1,2,4-Trimethylbenzene; ammonia at 100℃; for 50h; Product distribution / selectivity; | 96.2% |
| With hydrogen; Ni(NO3)2, Cu(NO3)2, Cr(NO3)3, Na2CO3; calcined at 380 deg C for 18 h; pretreatment is given in full text In 1,2,4-Trimethylbenzene; ammonia at 80℃; for 24h; Product distribution / selectivity; | 95.2% |

| Conditions | Yield |
|---|---|
| With Co-doped zirconium dioxide; ammonia; hydrogen; N-butylamine In methanol at 99.84℃; for 10h; Autoclave; chemoselective reaction; | 97% |
-
-
626-17-5
benzene-1,3-dicarbonitrile

-
A
-
1477-55-0
1,3-di(aminomethyl)benzene

-
B
-
100-81-2
3-methyl-benzenemethanamine

| Conditions | Yield |
|---|---|
| With hydrogen; Ni(NO3)2, Cu(NO3)2, Cr(NO3)3, Na2CO3; calcined at 380 deg C for 18 h; pretreatment is given in full text In 1,2,4-Trimethylbenzene; ammonia at 80℃; for 24h; Product distribution / selectivity; | A 96.8% B 0.1% |
| With hydrogen; Ni(NO3)2, Cu(NO3)2, Cr(NO3)3, Na2CO3; calcined at 380 deg C for 18 h; activated with H2 In 1,2,4-Trimethylbenzene; ammonia at 80℃; for 24h; Product distribution / selectivity; | A 92.1% B 0.1% |

| Conditions | Yield |
|---|---|
| With ammonia; hydrogen; catalyst A In mata-xylene at 80 - 110℃; Conversion of starting material; | A 93.1% B 5.5% |
-
-
626-17-5
benzene-1,3-dicarbonitrile

-
A
-
1477-55-0
1,3-di(aminomethyl)benzene

-
B
-
10406-24-3
3-(aminomethyl)benzonitrile

| Conditions | Yield |
|---|---|
| With hydrogen; nickel catalyst "NDHT"; pretreatment is given in full text In methanol; ammonia at 65℃; for 4h; Product distribution / selectivity; | A 92.8% B 0.2% |
| With hydrogen; Pd-alumina In ammonia; 1,3,5-trimethyl-benzene at 50℃; under 36753.7 Torr; | A 7.7% B 87.3% |
| With hydrogen; nickel catalyst "NDHT" In methanol; ammonia at 65℃; for 4h; Product distribution / selectivity; autoclave; | A 84.8% B 0.2% |
-
-
626-17-5
benzene-1,3-dicarbonitrile

-
-
10406-24-3
3-(aminomethyl)benzonitrile

-
-
1477-55-0
1,3-di(aminomethyl)benzene

| Conditions | Yield |
|---|---|
| With hydrogen; catalyst A In ammonia; 1,3,5-trimethyl-benzene at 50℃; under 36753.7 Torr; | 91.1% |
| With hydrogen; Ni-diatomaceous earth In ammonia; 1,3,5-trimethyl-benzene at 50℃; under 36753.7 Torr; | 89.4% |
-
-
626-17-5
benzene-1,3-dicarbonitrile

-
-
7664-41-7
ammonia

-
-
108-38-3
m-xylene

-
A
-
1477-55-0
1,3-di(aminomethyl)benzene

-
B
-
10406-24-3
3-(aminomethyl)benzonitrile

| Conditions | Yield |
|---|---|
| With hydrogen; Ni-3266E manufactured by Harshaw Co., Ltd.; nickel content: about 50percent at 55℃; under 112511 Torr; Product distribution / selectivity; tube reactor; feed rate = 1.5 t/h; supplying hydrogen rate = 100 Nm3/h; | A 91% B 0.1% |
| With hydrogen; catalyst A at 55℃; under 52505.3 Torr; Product distribution / selectivity; tube reactor; feed rate = 32 g/h; supplying hydrogen rate = 20 NL/h; | A 90.9% B 0.1% |


| Conditions | Yield |
|---|---|
| Stage #1: 3-(chloromethyl)benzyl chloride With ammonia In methanol at 50℃; for 6h; Stage #2: With sodium hydroxide In water pH=13; Solvent; | 89% |

| Conditions | Yield |
|---|---|
| 81.7% | |
| 79.5% |

| Conditions | Yield |
|---|---|
| With (carbonyl)(chloro)(hydrido)tris(triphenylphosphine)ruthenium(II); ammonia; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In tert-Amyl alcohol at 150℃; for 20h; Inert atmosphere; Cooling; | 70% |
| With (carbonyl)(chloro)(hydrido)tris(triphenylphosphine)ruthenium(II); ammonia; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In tert-Amyl alcohol at 150℃; under 12001.2 Torr; for 20h; Inert atmosphere; Autoclave; chemoselective reaction; | 70 %Chromat. |
-
-
626-17-5
benzene-1,3-dicarbonitrile

-
-
74-89-5
methylamine

-
A
-
1477-55-0
1,3-di(aminomethyl)benzene

-
B
-
85067-98-7
N,N'-(1,3-phenylenedimethylidyne)bis(methanamine)

| Conditions | Yield |
|---|---|
| With hydrogen; Raney Nickel In tetrahydrofuran at 125℃; under 19393.6 Torr; for 16.6667h; | A 7% B 45.3% C 14.3% |


| Conditions | Yield |
|---|---|
| With hydrogen In tetrahydrofuran at 125℃; under 19393.6 Torr; for 16.6667h; | 44.9% |
| With hydrogen; Rh/Al2O3 In tetrahydrofuran at 125℃; under 19393.6 Torr; for 16.6667h; | |
| With hydrogen; palladium 10% on activated carbon In tetrahydrofuran at 125℃; under 19393.6 Torr; for 16.6667h; |
-
-
626-17-5
benzene-1,3-dicarbonitrile

-
-
74-89-5
methylamine

-
A
-
1477-55-0
1,3-di(aminomethyl)benzene

-
B
-
1035316-05-2
3-(methylaminomethyl)benzylamine

-
C
-
23399-62-4
N,N′-dimethyl-m-xylylenediamine

-
D
-
85067-98-7
N,N'-(1,3-phenylenedimethylidyne)bis(methanamine)

| Conditions | Yield |
|---|---|
| With hydrogen; Raney Nickel In tetrahydrofuran at 125℃; under 19393.6 Torr; for 19.6667h; | A 9.5% B 41.3% C 14.5% D 9.4% |
| With hydrogen; Raney nickel In tetrahydrofuran at 125℃; under 19393.6 Torr; for 19.6667h; |


-
-
1477-55-0
1,3-di(aminomethyl)benzene

| Conditions | Yield |
|---|---|
| With ammonia; hydrogen; catalyst A In m-xylene at 100 - 120℃; under 90009 Torr; Conversion of starting material; | 10.8% |
-
-
27199-63-9
N,N'-(1,3-phenylenebismethylene)bisphthalimide

-
-
1477-55-0
1,3-di(aminomethyl)benzene

| Conditions | Yield |
|---|---|
| With potassium hydroxide Erhitzen des Reaktionsprodukts mit wss.Salzsaeure; | |
| With hydrazine hydrate In ethanol for 4h; Reflux; | |
| With sodium hydroxide at 80 - 120℃; under 760.051 Torr; for 2h; Dean-Stark; | 51.5 g |

| Conditions | Yield |
|---|---|
| anschliessendes Erwaermen mit wss.NaOH; |
-
-
7647-01-0
hydrogenchloride

-
-
27199-63-9
N,N'-(1,3-phenylenebismethylene)bisphthalimide

-
A
-
1477-55-0
1,3-di(aminomethyl)benzene

-
B
-
88-99-3
benzene-1,2-dicarboxylic acid

| Conditions | Yield |
|---|---|
| at 200 - 220℃; |


-
-
1477-55-0
1,3-di(aminomethyl)benzene

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 200 - 220℃; |
-
-
10406-24-3
3-(aminomethyl)benzonitrile

-
-
1477-55-0
1,3-di(aminomethyl)benzene

| Conditions | Yield |
|---|---|
| With hydrogen; nickel/diatomaceous earth catalyst (nickel 50wtpercent) reduced with hydrogen at 200C at 100℃; under 30003 Torr; Product distribution / selectivity; |

-
-
1477-55-0
1,3-di(aminomethyl)benzene

| Conditions | Yield |
|---|---|
| With hydrogen; catalyst A In ammonia; xylene at 75℃; under 112511 Torr; Conversion of starting material; The continuous flow reaction .The concentration of benzamide compound-0.011%, benzoic acid compound-0.001%; | 90.9 %Chromat. |
| With hydrogen; catalyst A In ammonia; xylene at 90℃; under 112511 Torr; for 504h; Conversion of starting material; The continuous flow reaction .The concentration of bezamide compound-0.011%, benzoic acid compound-0.001%; | 90.9 %Chromat. |

-
A
-
1477-55-0
1,3-di(aminomethyl)benzene

-
B
-
10406-24-3
3-(aminomethyl)benzonitrile

| Conditions | Yield |
|---|---|
| With hydrogen; catalyst A In methanol; ammonia at 80℃; under 150015 Torr; Conversion of starting material; batch-wise method ; concentration benzamide-0.043w.%, benzoic acid-0.004w.%; | A 75.7 %Chromat. B 0.3 %Chromat. |

-
-
1477-55-0
1,3-di(aminomethyl)benzene

| Conditions | Yield |
|---|---|
| With hydrogen; catalyst A In ammonia; xylene at 75℃; under 112511 Torr; Conversion of starting material; The continuous flow reaction . The concentration of benzamide compound-0.063%, benzoic acid compound-0.001%; | 89.4 %Chromat. |
| With hydrogen; catalyst A In ammonia; xylene at 90℃; under 112511 Torr; for 432h; Conversion of starting material; The continuous flow reaction .The concentration of benzamide compound-0.063%, benzoic acid compound-0.001%; | 89.4 %Chromat. |

-
A
-
1477-55-0
1,3-di(aminomethyl)benzene

-
B
-
10406-24-3
3-(aminomethyl)benzonitrile

| Conditions | Yield |
|---|---|
| With hydrogen; catalyst A In methanol; ammonia at 80℃; under 150015 Torr; Conversion of starting material; The butch-wize method.The concentration of bezamide-0.30%, benoic acid-0.004%; | A 74.8 %Chromat. B 0.3 %Chromat. |

-
A
-
1477-55-0
1,3-di(aminomethyl)benzene

-
B
-
10406-24-3
3-(aminomethyl)benzonitrile

| Conditions | Yield |
|---|---|
| With hydrogen; catalyst A In methanol; ammonia at 80℃; under 150015 Torr; Conversion of starting material; The batch-wize method; | A 70.2 %Chromat. B 0.5 %Chromat. |

-
A
-
1477-55-0
1,3-di(aminomethyl)benzene

-
B
-
10406-24-3
3-(aminomethyl)benzonitrile

| Conditions | Yield |
|---|---|
| With hydrogen; catalyst A In methanol; ammonia at 80℃; under 150015 Torr; Conversion of starting material; The batch-wise method; | A 69.9 %Chromat. B 0.9 %Chromat. |

-
-
1477-55-0
1,3-di(aminomethyl)benzene

| Conditions | Yield |
|---|---|
| With hydrogen; catalyst A In ammonia; xylene at 75℃; under 112511 Torr; Conversion of starting material; The continuous flow reaction .The concentration of benzamide compound-0.114%, benzoic acid compound-0.002%; | 87.8 %Chromat. |
| With hydrogen; catalyst A In ammonia; xylene at 90℃; under 112511 Torr; for 288h; Conversion of starting material; The continuous flow reaction .The concentration of benzamide compound-0.114%, benzoic acid compound-0.002%; | 87.8 %Chromat. |

-
-
1477-55-0
1,3-di(aminomethyl)benzene

| Conditions | Yield |
|---|---|
| With hydrogen; catalyst A In ammonia; xylene at 75℃; under 112511 Torr; Conversion of starting material; The continuous flow reaction .The concentration of benzamide compound-0.012%, bezoic acid compound-0.012%; | 88.8 %Chromat. |
| With hydrogen; catalyst A In ammonia; xylene at 90℃; under 112511 Torr; for 408h; Conversion of starting material; The continuous flow reaction .The concentration of benzamide compound-0.012%, benzoic acid compound-0.012%; | 88.8 %Chromat. |

-
A
-
1477-55-0
1,3-di(aminomethyl)benzene

-
B
-
10406-24-3
3-(aminomethyl)benzonitrile

| Conditions | Yield |
|---|---|
| With hydrogen; catalyst A In methanol; ammonia at 80℃; under 150015 Torr; Conversion of starting material; The batch-wise method .The concentration of benzamide0.043%, benzoic acid-0.043%; | A 74.1 %Chromat. B 0.2 %Chromat. |
| With hydrogen; sodium hydroxide; PDC-3000 In ammonia at 50℃; under 150015 Torr; for 0.5h; Product distribution / selectivity; The batch-wise method . The concentration of benzamide compound-0.043%, benzoic acid compound-0.043%; | A 8.4 %Chromat. B 76 %Chromat. |
-
-
1477-55-0
1,3-di(aminomethyl)benzene

-
-
61696-55-7, 73891-16-4, 119067-16-2
(2S,3S,11S,12S)-1,4,7,10,13,16-Hexaoxa-cyclooctadecane-2,3,11,12-tetracarbonyl tetrachloride

-
-
129166-26-3
C32H40N4O10

| Conditions | Yield |
|---|---|
| In toluene | 100% |
-
-
1477-55-0
1,3-di(aminomethyl)benzene

-
-
887129-89-7
7,11,15,28-tetrakis(bromomethyl)-1,21,23,25-tetramethyl-2,20:3,19-dimetheno-1H,21H,23H,25H-bis<1,3>dioxocino<5,4-i:5',4'-i'>benzo<1,2-d:5,4-d'>bis<1,3>benzodioxocin

| Conditions | Yield |
|---|---|
| at 100℃; | 100% |
-
-
1477-55-0
1,3-di(aminomethyl)benzene

-
-
682-30-4
Diethyl vinylphosphonate

-
-
1073193-35-7
1,3-bis{(N,N-bis[(diethoxyphosphoryl)ethyl])aminomethyl}benzene

| Conditions | Yield |
|---|---|
| In water at 20℃; Michael condensation; | 100% |
| In water at 20℃; for 120h; Aza-Michael reaction; | 83% |
-
-
1477-55-0
1,3-di(aminomethyl)benzene

-
-
77123-57-0
4-[(trimethylsilyl)ethynyl]bezaldehyde

-
-
1219537-39-9
C32H36N2Si2

| Conditions | Yield |
|---|---|
| With magnesium sulfate In diethyl ether at 20℃; for 3h; | 100% |
-
-
869857-96-5
(2S,3S,4R,5S)-1-benzyloxycarbonyl-2-carboxy-5-methylpyrrolidine-3,4-diol

-
-
1477-55-0
1,3-di(aminomethyl)benzene

| Conditions | Yield |
|---|---|
| With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| In water | 100% |
-
-
1477-55-0
1,3-di(aminomethyl)benzene

-
-
26106-63-8
tetrahydrofuran-2,3,4,5-tetracarboxylic acid

| Conditions | Yield |
|---|---|
| In water | 100% |
-
-
1477-55-0
1,3-di(aminomethyl)benzene

-
-
2421-28-5
dianhydride of benzophenone-3,4,3',4'-tetracarboxy acid

| Conditions | Yield |
|---|---|
| In water for 0.5h; | 100% |
-
-
1477-55-0
1,3-di(aminomethyl)benzene

| Conditions | Yield |
|---|---|
| With hydrogenchloride In 1,4-dioxane; methanol for 0.166667h; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 1,3-di(aminomethyl)benzene With hydrogenchloride In water; 1,2-dichloro-benzene at 20℃; Autoclave; Large scale; Stage #2: phosgene In water; 1,2-dichloro-benzene at 145℃; for 2h; Autoclave; Large scale; | 99.9% |
| With picoline In dichloromethane at -5 - 140℃; for 4h; Reagent/catalyst; Temperature; | 98.3% |
| Stage #1: 1,3-di(aminomethyl)benzene With hydrogenchloride In chlorobenzene at 10℃; under 750.075 Torr; for 1.5h; Flow reactor; Large scale; Stage #2: phosgene In chlorobenzene at 131℃; under 750.075 Torr; for 3h; Temperature; Pressure; Solvent; Flow reactor; Large scale; | 98.5% |
-
-
1477-55-0
1,3-di(aminomethyl)benzene

-
-
617-09-4
di-o-tolyl carbonate

-
-
54772-36-0
1,3-bis-[(methoxycarbonylamino)-methyl]-benzene

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol | 99.7% |
-
-
1477-55-0
1,3-di(aminomethyl)benzene

-
-
1422-70-4
bis(2,2,3,3-tetrafluoropropyl) carbonate

-
-
1338600-43-3
m-xylylene bis(2,2,3,3-tetrafluoropropylcarbamate)

| Conditions | Yield |
|---|---|
| at 50℃; for 1h; | 99.4% |
-
-
1477-55-0
1,3-di(aminomethyl)benzene

-
-
652149-71-8
4-(4'-tert-butyloxycarbonylphenylethynyl)benzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 4-(4'-tert-butyloxycarbonylphenylethynyl)benzoic acid With oxalyl dichloride In dichloromethane; N,N-dimethyl-formamide Stage #2: 1,3-di(aminomethyl)benzene With triethylamine In dichloromethane Stage #3: With trifluoroacetic acid In dichloromethane | 99% |
-
-
1477-55-0
1,3-di(aminomethyl)benzene

-
-
106-89-8
epichlorohydrin

-
-
63738-22-7
N,N,N',N'-tetraglycidyl-m-xylylenediamine

| Conditions | Yield |
|---|---|
| With N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide In water; toluene at 90℃; for 1h; Product distribution / selectivity; Inert atmosphere; | 98.4% |
-
-
1477-55-0
1,3-di(aminomethyl)benzene

-
-
107-31-3
Methyl formate

-
-
616-38-6
carbonic acid dimethyl ester

-
-
54772-36-0
1,3-bis-[(methoxycarbonylamino)-methyl]-benzene

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol at 70℃; for 3h; | 98.4% |

-
-
1477-55-0
1,3-di(aminomethyl)benzene

-
-
616-38-6
carbonic acid dimethyl ester

-
-
54772-36-0
1,3-bis-[(methoxycarbonylamino)-methyl]-benzene

| Conditions | Yield |
|---|---|
| lipase from Candida antarctica In toluene at 70℃; for 60h; Product distribution / selectivity; | 98% |
| With lead(II) acetate trihydrate; zinc(II) acetate dihydrate In water at 180℃; under 2250.23 Torr; for 6h; Reagent/catalyst; Inert atmosphere; | 98.2% |
| With water; sodium methylate In methanol at 50℃; for 3.5h; | 97% |
| 79.3% |

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In chloroform at 20℃; for 24h; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: 1,3-di(aminomethyl)benzene; 4-(trifluoromethoxy)-2-formylphenylboronic acid In toluene at 50℃; for 12h; Stage #2: With sodium tetrahydroborate In toluene at 25℃; for 4h; Cooling with ice; | 98% |
-
-
1477-55-0
1,3-di(aminomethyl)benzene

-
-
616-02-4
citraconic acid anhydride

-
-
119462-56-5
1,3-bis(citraconimidomethyl)benzol

| Conditions | Yield |
|---|---|
| With acetic acid In toluene at 110℃; for 5h; Temperature; Reagent/catalyst; Solvent; | 97.5% |
| With acetic acid at 110℃; for 4h; Temperature; Time; | 92% |
-
-
1477-55-0
1,3-di(aminomethyl)benzene

-
-
98-88-4
benzoyl chloride

-
-
33891-00-8
N,N'-(1,3-phenylenebis(methylene))dibenzamide

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 0℃; for 3h; | 97% |
| With (+/-)-propylene oxide In tetrahydrofuran at 20℃; | 67% |
| With pyridine |
-
-
1477-55-0
1,3-di(aminomethyl)benzene

-
-
213273-03-1
4-oxo-2-(N-phenyl)amino-4H-chromene-3-carbaldehyde

| Conditions | Yield |
|---|---|
| In acetonitrile | 97% |
-
-
1477-55-0
1,3-di(aminomethyl)benzene

-
-
49568-76-5
3-benzoylquinoxalin-2(1H)-one

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 150℃; for 72h; | 97% |
-
-
1477-55-0
1,3-di(aminomethyl)benzene

-
-
85-44-9
phthalic anhydride

-
-
27199-63-9
N,N'-(1,3-phenylenebismethylene)bisphthalimide

| Conditions | Yield |
|---|---|
| In 1-methyl-pyrrolidin-2-one; xylene at 120℃; Product distribution / selectivity; | 97% |
| With acetic acid Heating; | 91% |
-
-
1477-55-0
1,3-di(aminomethyl)benzene

-
-
1521-38-6
2,3-dimethoxybenzoic acid

-
-
188746-15-8
N,N'-[1,3-phenylenebis(methylene)]bis-(2,3-dimethoxybenzamide)

| Conditions | Yield |
|---|---|
| Stage #1: 2,3-dimethoxybenzoic acid With oxalyl dichloride; N,N-dimethyl-formamide In dichloromethane Stage #2: 1,3-di(aminomethyl)benzene With triethylamine In dichloromethane for 18h; Further stages.; | 97% |
-
-
1477-55-0
1,3-di(aminomethyl)benzene

-
-
77123-55-8
3-((trimethylsilyl)ethynyl)benzaldehyde

-
-
1219537-45-7
C32H36N2Si2

| Conditions | Yield |
|---|---|
| With magnesium sulfate In diethyl ether at 20℃; for 3h; | 97% |
-
-
1477-55-0
1,3-di(aminomethyl)benzene

-
-
111477-43-1
N-(6-(bromomethyl)-2-pyridyl)pivalamide

-
-
1258528-12-9
C52H68N10O4

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile Reflux; | 97% |
-
-
1477-55-0
1,3-di(aminomethyl)benzene

-
-
90-02-8
salicylaldehyde

-
-
51540-97-7
2,2'-(1,3-phenylenebis(methylene))bis(azan-1-yl-1-ylidene)bis(methan-1-yl-1-ylidene)diphenol

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 0.166667h; Temperature; Microwave irradiation; Green chemistry; | 97% |
| In methanol for 3h; Schiff reaction; Heating; | 90% |
| With formic acid In ethanol for 10h; Reflux; | 75.2% |
| In methanol for 4h; Reflux; | 74% |
| In methanol for 2h; Reflux; |

| Conditions | Yield |
|---|---|
| In dichloromethane Inert atmosphere; Reflux; | 97% |
1,3-Bis(aminomethyl)benzene Consensus Reports
1,3-Bis(aminomethyl)benzene Standards and Recommendations
ACGIH TLV: TWA CL 0.1 mg/m3 (skin)
1,3-Bis(aminomethyl)benzene Specification
The 1,3-Bis(aminomethyl)benzene is an organic compound with the formula C8H12N2. The IUPAC name of this chemical is [3-(aminomethyl)phenyl]methanamine. With the CAS registry number 1477-55-0, it is also named as benzene-1,3-diyldimethanamine. The product's categories are Intermediates of Dyes and Pigments; Acetone series; Rubber & Plastic Auxiliary Agent. Besides, it is clear slightly yellow liquid, which should be stored in a closed cool and dry place. It is used as epoxy curing agent. It is also used as raw materials of photosensitive plastics, rubber chemicals, polyurethane resins, coatings and intermediates in organic synthesis.
Physical properties about 1,3-Bis(aminomethyl)benzene are: (1)ACD/LogP: -0.04; (2)ACD/LogD (pH 5.5): -4.14; (3)ACD/LogD (pH 7.4): -3.6; (4)ACD/BCF (pH 5.5): 1; (5)ACD/BCF (pH 7.4): 1; (6)ACD/KOC (pH 5.5): 1; (7)ACD/KOC (pH 7.4): 1; (8)#H bond acceptors: 2; (9)#H bond donors: 4; (10)#Freely Rotating Bonds: 4; (11)Polar Surface Area: 6.48 Å2; (12)Index of Refraction: 1.581; (13)Molar Refractivity: 43.15 cm3; (14)Molar Volume: 129.4 cm3; (15)Polarizability: 17.1×10-24cm3; (16)Surface Tension: 47 dyne/cm; (17)Density: 1.052 g/cm3; (18)Flash Point: 130.4 °C; (19)Enthalpy of Vaporization: 48.41 kJ/mol; (20)Boiling Point: 247 °C at 760 mmHg; (21)Vapour Pressure: 0.0263 mmHg at 25°C.
Preparation: this chemical can be prepared by isophthalonitrile. This reaction will need reagent H2, catalyst 65 percent Ni/(SiO2-Al2O3) and solvent liquid ammonia propan-2-ol. The reaction time is 60 min with reaction temperature of 170 °C. The yield is about 82%.
.gif)
Uses of 1,3-Bis(aminomethyl)benzene: it can be used to produce 1,3-bis-isothiocyanatomethyl-benzene at room temperature. It will need reagent NEt3 and solvent CH2Cl2 with reaction time of 1.5 hours. The yield is about 51%.
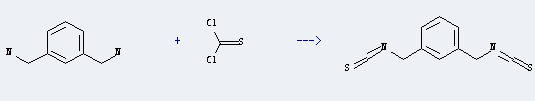
When you are using this chemical, please be cautious about it as the following:
It is harmful in contact with skin and if swallowed and toxic by inhalation. After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer). In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. Besides, this chemical is harmful to aquatic organisms, may cause long-term adverse effects in the aquatic environment. It can cause burns and may cause sensitisation by skin contact. When you are using it, wear suitable gloves and eye/face protection and avoid release to the environment. Refer to special instructions/safety data sheet. In case of accident or if you feel unwell seek medical advice immediately (show the label where possible).
You can still convert the following datas into molecular structure:
(1)SMILES: NCc1cccc(c1)CN
(2)InChI: InChI=1/C8H12N2/c9-5-7-2-1-3-8(4-7)6-10/h1-4H,5-6,9-10H2
(3)InChIKey: FDLQZKYLHJJBHD-UHFFFAOYAK
(4)Std. InChI: InChI=1S/C8H12N2/c9-5-7-2-1-3-8(4-7)6-10/h1-4H,5-6,9-10H2
(5)Std. InChIKey: FDLQZKYLHJJBHD-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rabbit | LD50 | skin | 2gm/kg (2000mg/kg) | "Documentation of the Threshold Limit Values and Biological Exposure Indices," 5th ed., Cincinnati, OH, American Conference of Governmental Industrial Hygienists, Inc., 1986Vol. 5, Pg. 638, 1986. | |
| rat | LC50 | inhalation | 700ppm/1H (700ppm) | SENSE ORGANS AND SPECIAL SENSES: LACRIMATION: EYE LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | "Documentation of the Threshold Limit Values and Biological Exposure Indices," 5th ed., Cincinnati, OH, American Conference of Governmental Industrial Hygienists, Inc., 1986Vol. 5, Pg. 638, 1986. |
| rat | LD50 | oral | 930mg/kg (930mg/kg) | "Documentation of the Threshold Limit Values and Biological Exposure Indices," 5th ed., Cincinnati, OH, American Conference of Governmental Industrial Hygienists, Inc., 1986Vol. 5, Pg. 638, 1986. |
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 1477-57-2
- 147762-53-6
- 1477-68-5
- 147769-93-5
- 147770-06-7
- 14777-27-6
- 147779-25-7
- 147780-39-0
- 147782-19-2
- 14779-17-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View