-
Name
1,3-Dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone
- EINECS 230-625-6
- CAS No. 7226-23-5
- Article Data21
- CAS DataBase
- Density 1.024 g/cm3
- Solubility Soluble in water and most organic solvents
- Melting Point -20 °C
- Formula C6H12N2O
- Boiling Point 240.2 °C at 760 mmHg
- Molecular Weight 128.174
- Flash Point 85.3 °C
- Transport Information
- Appearance clear colorless to slightly yellowish liquid
- Safety 23-26-36/37/39-45
- Risk Codes 22-36-43-62-41
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms 1,3-Dimethyl-2-oxohexahydropyrimidine;1,3-Dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidone;1,3-Dimethyl-3,4,5,6-tetrahydro-2-pyrimidinone;1,3-Dimethylpropyleneurea;DMPU (solvent);N,N'-Dimethyl-1,3-propanediamine cyclic urea;N,N'-Dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone;N,N'-Dimethyl-N,N'-propyleneurea;N,N'-Dimethyltrimethyleneurea;
- PSA 23.55000
- LogP 0.24950
Synthetic route
-
-
201230-82-2
carbon monoxide

-
-
111-33-1
1,3-bis(methylamino)propane

-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

| Conditions | Yield |
|---|---|
| Stage #1: carbon monoxide; 1,3-bis(methylamino)propane With selenium at 20℃; under 750.075 Torr; for 2h; neat (no solvent); Stage #2: With oxygen at 20℃; under 750.075 Torr; for 1h; neat (no solvent); | 94% |
| With iodine; potassium carbonate; tungsten hexacarbonyl In dichloromethane at 20℃; under 76005.1 Torr; | 52% |
-
-
53527-07-4
β-(4-hydroxyphenyl)ethyl iodoacetamide


-
A
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

-
B
-
55710-99-1
β-(4-hydroxyphenyl)ethyl-2-aminoacetamide

| Conditions | Yield |
|---|---|
| With diethylenetriaminopentaacetic acid In aq. buffer at 37℃; for 7h; pH=7.4; Kinetics; Solvent; pH-value; | A n/a B n/a C 90% D n/a |

-
-
2055-46-1
3,4,5,6-tetrahydropyrimidine-2-thione

-
-
74-88-4
methyl iodide

-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

| Conditions | Yield |
|---|---|
| With sodium hydroxide; N-benzyl-N,N,N-triethylammonium chloride for 5h; Heating; | 81% |
| With 18-crown-6 ether; N-benzyl-N,N,N-triethylammonium chloride for 2h; Heating; | 81% |
-
-
1852-17-1
3,4,5,6-tetrahydropyrimidin-2(1H)-one

-
-
77-78-1
dimethyl sulfate

-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

| Conditions | Yield |
|---|---|
| With N-benzyl-N,N,N-triethylammonium chloride; potassium carbonate In 1,4-dioxane at 60℃; for 5h; | 78.8% |

-
-
109-77-3
malononitrile

-
A
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

-
B
-
55727-22-5
(1,3-dimethyl-tetrahydro-pyrimidin-2-ylidene)-malononitrile

| Conditions | Yield |
|---|---|
| With dimethylaminomethyl-polystyrene In acetonitrile at 81℃; for 8.5h; | A n/a B 78% |

-
-
124-38-9
carbon dioxide

-
-
142-28-9
1,3-Dichloropropane

-
-
74-89-5
methylamine

-
A
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

-
B
-
96690-06-1
3-methyl-1,3-oxazinan-2-one

| Conditions | Yield |
|---|---|
| In water at 120℃; under 3750.38 Torr; for 2h; | A 64% B 15% |

-
-
123183-72-2
N-(tert-butyl)oxycarbonyl-N,N’-trimethyl-1,3-diaminopropane

-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: N,N-dimethyl-formamide / 5 h / 0 - 20 °C 2: dichloromethane / 1 h / 20 °C / Inert atmosphere 3: 37 °C / pH 5.5 View Scheme | |
| Multi-step reaction with 3 steps 1: triethylamine / N,N-dimethyl-formamide / 1 h / 0 - 20 °C / Inert atmosphere 2: dichloromethane / 1 h / 20 °C / Inert atmosphere 3: 37 °C / pH 5.5 View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: tetrahydrofuran / 22 h / 0 - 20 °C / Inert atmosphere 2: N,N-dimethyl-formamide / 5 h / 0 - 20 °C 3: dichloromethane / 1 h / 20 °C / Inert atmosphere 4: 37 °C / pH 5.5 View Scheme | |
| Multi-step reaction with 4 steps 1: tetrahydrofuran / 22 h / 0 - 20 °C / Inert atmosphere 2: triethylamine / N,N-dimethyl-formamide / 1 h / 0 - 20 °C / Inert atmosphere 3: dichloromethane / 1 h / 20 °C / Inert atmosphere 4: 37 °C / pH 5.5 View Scheme |

-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

| Conditions | Yield |
|---|---|
| at 37℃; pH=5.5; |

-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

| Conditions | Yield |
|---|---|
| at 37℃; pH=5.5; |
-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

-
-
73183-34-3
bis(pinacol)diborane

-
-
1391930-67-8
1-methyl-3-((4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)methyl)tetrahydropyrimidin-2(1H)-one

| Conditions | Yield |
|---|---|
| With C25H36O2P2Ru In neat (no solvent) at 120℃; for 24h; Catalytic behavior; Green chemistry; | 98% |
| With (1,5-cyclooctadiene)(methoxy)iridium(I) dimer; 8-(diisopropylsilyl)quinoline In 1,4-dioxane at 120℃; for 20h; Inert atmosphere; Sealed tube; | 96% |
| With methoxy(cyclooctadiene)rhodium(I) dimer In hexane at 40℃; for 1h; Inert atmosphere; Sealed tube; | 75% |
-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

-
-
94138-28-0
samarium diiodide bis(tetrahydrofuran)

| Conditions | Yield |
|---|---|
| In tetrahydrofuran 6-fold excess of ligand added to soln. of Sm complex in THF at room temp. under Ar; left to stand for 2 h; evapd., washed with toluene; elem. anal.; X-ray detn.; | 97% |
-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

-
-
73183-34-3
bis(pinacol)diborane

-
A
-
1391930-67-8
1-methyl-3-((4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)methyl)tetrahydropyrimidin-2(1H)-one

| Conditions | Yield |
|---|---|
| With methoxy(cyclooctadiene)rhodium(I) dimer In hexane at 25℃; for 1h; Inert atmosphere; regioselective reaction; | A 97% B 7% |
| With catalyst:[Rh(OCH3)(C8H12)]2 and silicaphosphine In hexane diboron, amide, catalyst:(Rh(OCH3)(C8H12))2, silica-supported triarylphosphine, hexane, 25°C, 1 h or 70°C 3 h; | A 85% B 2% |

-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

-
-
14965-49-2
methylamine hydroiodide

| Conditions | Yield |
|---|---|
| at 80℃; for 0.5h; | 96% |

-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

-
-
354-64-3
Pentafluoroethyl iodide

-
-
557-20-0
diethylzinc

| Conditions | Yield |
|---|---|
| In hexane; toluene at -35 - -5℃; for 24h; Inert atmosphere; | 95% |
| In hexane at -60 - 0℃; for 48h; Inert atmosphere; | 86% |

-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

-
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
-
557-20-0
diethylzinc

| Conditions | Yield |
|---|---|
| In hexane at -60 - -20℃; for 48h; Inert atmosphere; | 95% |
| In hexane; toluene at -35 - -5℃; for 24h; Inert atmosphere; | 93% |
| In hexane; toluene at -41 - -5℃; for 12h; Inert atmosphere; | 92% |

-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

-
-
354-64-3
Pentafluoroethyl iodide

-
-
557-20-0
diethylzinc

| Conditions | Yield |
|---|---|
| In hexane; toluene at -35 - -5℃; for 24h; Inert atmosphere; | 95% |

-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

-
-
1493-03-4
iododifluoromethane

-
-
557-20-0
diethylzinc

| Conditions | Yield |
|---|---|
| In hexane; pentane at -20 - 20℃; Inert atmosphere; Glovebox; | 94% |

-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

-
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
-
557-20-0
diethylzinc

| Conditions | Yield |
|---|---|
| In hexane; toluene at -35 - -5℃; for 24h; Inert atmosphere; | 93% |

-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

-
-
172747-89-6
triphenyltin dimesylamide

| Conditions | Yield |
|---|---|
| In acetonitrile N2 atm.; equimolar ratio, stirring (20°C), refluxing (2 h); evapn., addn. of Et2O, digestion, filtn., washing (Et2O), drying (vac.);elem. anal.; | 92% |
-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

-
-
375-80-4
dodecafluoro-1,6-diiodohexane

-
-
557-20-0
diethylzinc

| Conditions | Yield |
|---|---|
| Stage #1: dodecafluoro-1,6-diiodohexane; diethylzinc In hexane; pentane at -78 - 20℃; Inert atmosphere; Glovebox; Stage #2: 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone In hexane; pentane at 20℃; Inert atmosphere; Glovebox; | 92% |

-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

-
-
1493-03-4
iododifluoromethane

-
-
557-20-0
diethylzinc

| Conditions | Yield |
|---|---|
| In hexane Inert atmosphere; | 92% |
| In hexane; pentane at -20 - 20℃; for 2h; | 89% |
| In hexane; pentane at -20 - 20℃; for 2h; | 89% |

-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

-
-
136-95-8
2-amino-benzthiazole

-
-
144467-83-4
Benzothiazol-2-ylamine; compound with 1,3-dimethyl-tetrahydro-pyrimidin-2-one

| Conditions | Yield |
|---|---|
| In toluene at 0℃; for 24h; | 91% |
-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

-
-
94348-89-7
{(η5-C5Me5)2YCl}2

-
-
431887-85-3
(C5(CH3)5)2YCl((CH2)3(NCH3)2CO)

| Conditions | Yield |
|---|---|
| In benzene (N2); addn. of a soln. of ligand in benzene to a slurry of yttrium complex in benzene, stirring for 1 h; evapn.; elem. anal.; | 90% |
-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

-
-
2314-97-8
iodotrifluoromethane

-
-
557-20-0
diethylzinc

| Conditions | Yield |
|---|---|
| In hexane at -60 - -20℃; for 48h; Inert atmosphere; | 90% |

-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

-
-
2314-97-8
iodotrifluoromethane

-
-
557-20-0
diethylzinc

| Conditions | Yield |
|---|---|
| In hexane at -60 - -20℃; for 72h; Inert atmosphere; | 90% |

-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

-
-
2314-97-8
iodotrifluoromethane

-
-
557-20-0
diethylzinc

| Conditions | Yield |
|---|---|
| In hexane at -60 - -20℃; for 72h; Inert atmosphere; | 90% |

-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

-
-
86-74-8
9H-carbazole

-
-
2236-52-4
2,2'-diiodobiphenyl

-
-
207447-27-6
9-(4'-iodine-[1,1'-biphenyl]-4-yl)-9H-carbazole

| Conditions | Yield |
|---|---|
| With potassium carbonate; 18-crown-6 ether; copper(I) iodide In hexane; water; toluene | 89% |

-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

-
-
423-39-2
1-iodo-2,2,3,3,4,4,5,5,5-nonafluorobutane

-
-
557-20-0
diethylzinc

| Conditions | Yield |
|---|---|
| In toluene at -35 - -5℃; for 24h; Inert atmosphere; | 89% |

-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

-
-
423-39-2
1-iodo-2,2,3,3,4,4,5,5,5-nonafluorobutane

-
-
557-20-0
diethylzinc

| Conditions | Yield |
|---|---|
| In hexane; toluene at -35 - -5℃; for 24h; Inert atmosphere; | 89% |
| In hexane at -35 - -5℃; for 24h; Inert atmosphere; | 89% |
| In hexane; toluene at -41 - -5℃; for 12h; Inert atmosphere; | 88% |

-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

-
-
355-43-1
n-perfluorohexyl iodide

-
-
557-20-0
diethylzinc

| Conditions | Yield |
|---|---|
| In hexane at -60 - 0℃; for 48h; Inert atmosphere; | 88% |
| In hexane; toluene at -35 - -5℃; for 24h; Inert atmosphere; | 88% |
| In hexane; toluene at -41 - -5℃; for 12h; Inert atmosphere; | 87% |

-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

-
-
355-43-1
n-perfluorohexyl iodide

-
-
557-20-0
diethylzinc

| Conditions | Yield |
|---|---|
| In hexane; toluene at -35 - -5℃; for 24h; Inert atmosphere; | 88% |

-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

-
-
3056-17-5
stavudin

-
-
938050-90-9
2',3'-didehydro-3'-deoxythymidine N,N-dimethyl propylene urea solvate

| Conditions | Yield |
|---|---|
| In acetone at 56℃; for 0.25 - 0.5h; Product distribution / selectivity; Heating / reflux; | 87.5% |
| In acetone at 6 - 56℃; for 0.25h; Product distribution / selectivity; | 87.5% |
| In isopropyl alcohol at 70 - 75℃; for 0.25h; Product distribution / selectivity; | 86.7% |
-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

-
-
172747-89-6
triphenyltin dimesylamide

| Conditions | Yield |
|---|---|
| In acetonitrile N2 atm.; 2 equiv. of ligand, stirring (20°C), refluxing (2 h); evapn., addn. of Et2O, digestion, filtn., washing (Et2O), drying (vac.);elem. anal.; | 87% |
-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

-
-
1356922-21-8
3-(3-bromophenyl)-1,2,4-triazolo[3,4-a]isoquinoline

-
-
86-74-8
9H-carbazole

-
-
1356922-22-9
3-[3-(9H-carbazol-9-yl)phenyl]-1,2,4-triazolo[3,4-a]isoquinoline

| Conditions | Yield |
|---|---|
| With sodium bicarbonate; potassium carbonate; copper(I) iodide; 18-crown-6 ether In chloroform | 86% |

-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

| Conditions | Yield |
|---|---|
| In tetrahydrofuran Ar-atmosphere; addn. of 6 equiv. ligand to metal salt, standing for 1 h; solvent removal (reduced pressure); | 85% |
-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

| Conditions | Yield |
|---|---|
| at 90℃; for 0.5h; | 85% |

-
-
4076-36-2
5-methyl-1,2,3,4-tetrazole

-
-
7226-23-5
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone

-
-
64-17-5
ethanol

| Conditions | Yield |
|---|---|
| at 120℃; for 72h; Sealed tube; | 84% |

1,3-Dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone Specification
The IUPAC name of Dimethylpropyleneurea is 1,3-dimethyl-1,3-diazinan-2-one. With the CAS registry number 7226-23-5 and EINECS 230-625-6, it is also named as 2(1H)-Pyrimidinone, tetrahydro-1,3-dimethyl-. The classification codes are Drug / Therapeutic Agent; TSCA Flag P [A commenced PMN (Premanufacture Notice) substance]. It is clear colorless to slightly yellowish liquid which is soluble in water and most organic solvents. Additionally, this chemical should be sealed in the container and stored in the cool and dry place.
The other characteristics of this product can be summarized as: (1)ACD/LogP: 0.36; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.36; (4)ACD/LogD (pH 7.4): 0.36; (5)ACD/BCF (pH 5.5): 1.11; (6)ACD/BCF (pH 7.4): 1.11; (7)ACD/KOC (pH 5.5): 37.53; (8)ACD/KOC (pH 7.4): 37.53; (9)#H bond acceptors: 3; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 0; (12)Polar Surface Area: 23.55 Å2; (13)Index of Refraction: 1.473; (14)Molar Refractivity: 35.12 cm3; (15)Molar Volume: 125 cm3; (16)Polarizability: 13.92×10-24 cm3; (17)Surface Tension: 32.4 dyne/cm; (18)Density: 1.024 g/cm3; (19)Flash Point: 85.3 °C; (20)Enthalpy of Vaporization: 47.71 kJ/mol; (21)Boiling Point: 240.2 °C at 760 mmHg; (22)Vapour Pressure: 0.0386 mmHg at 25°C.
Preparation of Dimethylpropyleneurea: It can be obtained by tetrahydro-pyrimidin-2-one and sulfuric acid dimethyl ester. This reaction needs reagents K2CO3, TEBA and solvent dioxane at temperature of 60 °C. The reaction time is 5 hours. The yield is 78.8%.
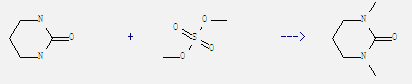
Uses of Dimethylpropyleneurea: It is a cyclic urea sometimes used as a polar, aprotic organic solvent. And it can react with trifluoro-methanesulfonic acid anhydride to get 2,2'-oxy-bis(1,3-dimethyl-tetrahydropyrimidinium) bis(trifluoromethanesulfonate). This reaction needs solvent CH2Cl2 by heating. The reaction time is 48 hours. The yield is 50%.
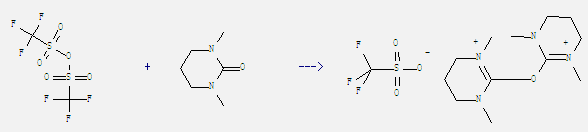
When you are using this chemical, please be cautious about it as the following:
It is not only harmful if swallowed, but also irritating to eyes. And it may cause sensitization by skin contact. So people should not breathe vapour. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. If you want to contact this product, you must wear suitable protective clothing, gloves and eye/face protection. In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
People can use the following data to convert to the molecule structure.
1. SMILES:O=C1N(C)CCCN1C
2. InChI:InChI=1/C6H12N2O/c1-7-4-3-5-8(2)6(7)9/h3-5H2,1-2H3
3. InChIKey:GUVUOGQBMYCBQP-UHFFFAOYAB
The following are the toxicity data which has been tested.
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 1300mg/kg (1300mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: ATAXIA LUNGS, THORAX, OR RESPIRATION: RESPIRATORY STIMULATION | Journal of Medicinal Chemistry. Vol. 11, Pg. 214, 1968. |
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 72263-05-9
- 7226-44-0
- 72264-81-4
- 72264-84-7
- 7226-87-1
- 72269-48-8
- 72269-52-4
- 72269-53-5
- 72269-57-9
- 72269-92-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View