-
Name
1,3-Dimethyladamantane
- EINECS 211-870-8
- CAS No. 702-79-4
- Article Data60
- CAS DataBase
- Density 0.972 g/cm3
- Solubility Soluble in organic solvents,insoluble in water.
- Melting Point -30 °C
- Formula C12H20
- Boiling Point 199.9 °C at 760 mmHg
- Molecular Weight 164.291
- Flash Point 52.8 °C
- Transport Information
- Appearance clear colourless liquid
- Safety 16
- Risk Codes 10
-
Molecular Structure
-
Hazard Symbols
 F
F
- Synonyms Adamantane,1,3-dimethyl- (6CI,8CI);1,3-Dimethyltricyclo[3.3.1.13,7]decane;1,3-dimethyl-adamantane;Tricyclo[3.3.1.13,7]decane,1,3-dimethyl-;
- PSA 0.00000
- LogP 3.61280
Synthetic route

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile); tri-n-butyl-tin hydride at 90℃; for 1h; Solvent; Barton-McCombie Deoxygenation; Inert atmosphere; | 100% |
| With decane; 2,2’-azobis(4-methoxy-2,4-dimethyl)valeronitrile; tri-n-butyl-tin hydride In toluene at 80℃; for 0.0166667h; | 99 % Chromat. |
| Multi-step reaction with 2 steps 1: 98 percent / aq. HCl / dimethylformamide / 1.5 h 2: H2SO4 (91.5percent) / 72 h / Ambient temperature; var. of conc.of H2SO4, and var. of reaction time View Scheme |
-
-
702-77-2
1-bromo-3-methyladamantane

-
-
75-16-1
methylmagnesium bromide

-
A
-
702-79-4
1,3-dimethyladamantane

-
-
768-91-2
1-methyl-adamantane

| Conditions | Yield |
|---|---|
| In dibutyl ether at 85℃; | A 99% B 1% |

-
-
941-37-7
3,5-dimethyl-1-bromoadamantane

-
-
75-16-1
methylmagnesium bromide

-
A
-
702-79-4
1,3-dimethyladamantane

-
B
-
707-35-7
1,3,5-trimethyladamantane

| Conditions | Yield |
|---|---|
| In dibutyl ether at 95℃; | A 2% B 98% |


| Conditions | Yield |
|---|---|
| With aluminium trichloride at 69.85℃; for 50h; | 90% |
| With hydrogen fluoride; boron trifluoride Temperature; Autoclave; | 75% |
| With Y-zeolite(Na/H 0.97) In hexane at 300℃; for 10h; Concentration; Temperature; Time; Autoclave; Sealed tube; | 65% |
-
-
281-23-2
adamantane

-
-
74-88-4
methyl iodide

-
A
-
702-79-4
1,3-dimethyladamantane

-
-
768-91-2
1-methyl-adamantane

| Conditions | Yield |
|---|---|
| With Rh(PPh3)3Cl In dichloromethane at 150℃; for 3h; | A 79% B 21% |


| Conditions | Yield |
|---|---|
| With hydrogen fluoride; boron trifluoride at 100℃; for 4h; Temperature; Autoclave; | A 77% B 15% |
-
-
26919-42-6
(3,5-dimethyl-adamantan-1-yl)-methanol

-
-
702-79-4
1,3-dimethyladamantane

| Conditions | Yield |
|---|---|
| With cerium(III) chloride; 9,10-diphenylanthracene; 1,2-bis(2,4,6-triisopropylphenyl)disulfane; tetrabutyl-ammonium chloride In acetonitrile for 24h; Irradiation; Inert atmosphere; | 69% |
-
-
58865-53-5
(1α,4α,4aβ,5α,8α,8aβ)-decahydro-1,4:5,8-dimethanonaphthalene

-
A
-
702-79-4
1,3-dimethyladamantane

-
B
-
770-69-4
1-ethyladamantane

-
C
-
16267-35-9
1,4-dimethyladamantane

| Conditions | Yield |
|---|---|
| With Y-zeolite(Na/H 0.97) In hexane at 300℃; for 10h; Autoclave; Sealed tube; | A 67% B 6% C 22% |

-
-
876-53-9
1,3-dibromoadamantane

-
-
75-16-1
methylmagnesium bromide

-
A
-
702-79-4
1,3-dimethyladamantane

-
B
-
281-23-2
adamantane

-
-
768-91-2
1-methyl-adamantane

| Conditions | Yield |
|---|---|
| In dibutyl ether at 140℃; | A 52% B 4% C 44% |

-
-
876-53-9
1,3-dibromoadamantane

-
-
75-16-1
methylmagnesium bromide

-
A
-
702-79-4
1,3-dimethyladamantane

-
B
-
281-23-2
adamantane

-
C
-
702-77-2
1-bromo-3-methyladamantane

| Conditions | Yield |
|---|---|
| In dibutyl ether at 140℃; | A 52% B 4% C 44% |

-
-
876-53-9
1,3-dibromoadamantane

-
A
-
702-79-4
1,3-dimethyladamantane

-
B
-
281-23-2
adamantane

-
-
768-91-2
1-methyl-adamantane

| Conditions | Yield |
|---|---|
| With methylmagnesium bromide In dibutyl ether at 140℃; | A 52% B 4% C 44% |

-
-
58865-53-5
(1α,4α,4aβ,5α,8α,8aβ)-decahydro-1,4:5,8-dimethanonaphthalene

-
A
-
702-79-4
1,3-dimethyladamantane

-
B
-
16207-81-1
1,2-dimethyladamantane

-
C
-
770-69-4
1-ethyladamantane

-
D
-
16267-35-9
1,4-dimethyladamantane

| Conditions | Yield |
|---|---|
| With Y-zeolite(Na/H 0.4) In hexane at 300℃; for 30h; Concentration; Time; | A 49% B 9% C 11% D 24% |
| With Y-zeolite(Na/H 0.4) In hexane at 300℃; for 10h; Concentration; Time; | A 18% B 8% C 10% D 21% |

-
-
75-76-3
tetramethylsilane

-
-
768-95-6
1-adamanthanol

-
A
-
702-79-4
1,3-dimethyladamantane

-
B
-
281-23-2
adamantane

-
C
-
1687-36-1
1,3,5,7-tetramethyladamantane

-
D
-
707-35-7
1,3,5-trimethyladamantane

-
-
768-91-2
1-methyl-adamantane

| Conditions | Yield |
|---|---|
| With aluminum tri-bromide In dichloromethane at 20℃; for 5h; Product distribution; other time;; | A 33% B n/a C 10% D 29% E 13% |

-
-
75-76-3
tetramethylsilane

-
-
768-95-6
1-adamanthanol

-
A
-
702-79-4
1,3-dimethyladamantane

-
B
-
1687-36-1
1,3,5,7-tetramethyladamantane

-
C
-
707-35-7
1,3,5-trimethyladamantane

-
-
768-91-2
1-methyl-adamantane

| Conditions | Yield |
|---|---|
| With aluminum tri-bromide In dichloromethane at 20℃; for 5h; | A 33% B 10% C 29% D 13% |
| With aluminum tri-bromide |

-
-
1678-91-7
ethyl-cyclohexane

-
A
-
702-79-4
1,3-dimethyladamantane

-
B
-
1687-36-1
1,3,5,7-tetramethyladamantane

-
C
-
493-02-7
trans-Decalin

-
D
-
770-69-4
1-ethyladamantane

-
E
-
707-35-7
1,3,5-trimethyladamantane

-
-
768-91-2
1-methyl-adamantane

| Conditions | Yield |
|---|---|
| With aluminium trichloride; tertiary butyl chloride at 160℃; for 15h; Product distribution; | A 18.76% B 6.3% C 1.25% D 3.46% E 15.01% F 2.76% |

-
-
82166-21-0
methyl cyclohexane

-
A
-
702-79-4
1,3-dimethyladamantane

-
B
-
1687-36-1
1,3,5,7-tetramethyladamantane

-
C
-
493-02-7
trans-Decalin

-
D
-
770-69-4
1-ethyladamantane

-
E
-
707-35-7
1,3,5-trimethyladamantane

-
-
768-91-2
1-methyl-adamantane

| Conditions | Yield |
|---|---|
| With aluminium trichloride; tertiary butyl chloride at 160℃; for 15h; Product distribution; | A 10.78% B 7.79% C 4.7% D 4.25% E 17.49% F 1.5% |

-
-
58865-53-5
(1α,4α,4aβ,5α,8α,8aβ)-decahydro-1,4:5,8-dimethanonaphthalene

-
A
-
702-79-4
1,3-dimethyladamantane

-
B
-
16267-35-9
1,4-dimethyladamantane

| Conditions | Yield |
|---|---|
| With Y-zeolite(Na/H 0.4) In hexane at 300℃; for 10h; Concentration; Time; | A 17% B 15% |
| With Y-zeolite(Na/H 0.97) In hexane at 300℃; for 30h; Concentration; Time; | A 6% B 9% |
-
-
696-29-7
(1-methylethyl)-cyclohexane

-
A
-
702-79-4
1,3-dimethyladamantane

-
B
-
1687-36-1
1,3,5,7-tetramethyladamantane

-
C
-
493-02-7
trans-Decalin

-
D
-
770-69-4
1-ethyladamantane

-
E
-
707-35-7
1,3,5-trimethyladamantane

-
-
768-91-2
1-methyl-adamantane

| Conditions | Yield |
|---|---|
| With aluminium trichloride; tertiary butyl chloride at 160℃; for 15h; Product distribution; | A 8.98% B 7.37% C 7.47% D 2.04% E 13.24% F 1.59% |

-
-
1678-98-4
isobutylcyclohexane

-
A
-
702-79-4
1,3-dimethyladamantane

-
B
-
1687-36-1
1,3,5,7-tetramethyladamantane

-
C
-
493-02-7
trans-Decalin

-
D
-
770-69-4
1-ethyladamantane

-
E
-
707-35-7
1,3,5-trimethyladamantane

-
-
768-91-2
1-methyl-adamantane

| Conditions | Yield |
|---|---|
| With aluminium trichloride; tertiary butyl chloride at 160℃; for 15h; Product distribution; | A 8.23% B 8.26% C 9.82% D 0.59% E 10.13% F 1.33% |

-
-
768-90-1
1-Adamantyl bromide

-
-
75-76-3
tetramethylsilane

-
A
-
702-79-4
1,3-dimethyladamantane

-
B
-
281-23-2
adamantane

-
-
768-91-2
1-methyl-adamantane

| Conditions | Yield |
|---|---|
| With aluminium trichloride In dichloromethane at 20℃; for 0.166667h; Further byproducts given; | A 32 % Chromat. B 21 % Chromat. C 39 % Chromat. |
| With aluminum tri-bromide In dichloromethane at 20℃; for 1h; Further byproducts given; | A 35 % Chromat. B 13 % Chromat. C 37 % Chromat. |

-
-
768-90-1
1-Adamantyl bromide

-
-
75-76-3
tetramethylsilane

-
A
-
702-79-4
1,3-dimethyladamantane

-
B
-
281-23-2
adamantane

-
C
-
1687-36-1
1,3,5,7-tetramethyladamantane

-
D
-
707-35-7
1,3,5-trimethyladamantane

-
-
768-91-2
1-methyl-adamantane

| Conditions | Yield |
|---|---|
| With aluminum tri-bromide In dichloromethane at 20℃; for 0.166667h; Product distribution; other time; various ratios of the educts and AlBr3; AlCl3 instead AlBr3; |

-
-
768-90-1
1-Adamantyl bromide

-
-
75-76-3
tetramethylsilane

-
A
-
702-79-4
1,3-dimethyladamantane

-
B
-
281-23-2
adamantane

-
C
-
707-35-7
1,3,5-trimethyladamantane

-
-
768-91-2
1-methyl-adamantane

| Conditions | Yield |
|---|---|
| With aluminium trichloride In dichloromethane at 20℃; for 0.166667h; Yield given. Yields of byproduct given; |

-
-
768-90-1
1-Adamantyl bromide

-
-
75-76-3
tetramethylsilane

-
A
-
702-79-4
1,3-dimethyladamantane

-
B
-
1687-36-1
1,3,5,7-tetramethyladamantane

-
C
-
707-35-7
1,3,5-trimethyladamantane

-
-
768-91-2
1-methyl-adamantane

| Conditions | Yield |
|---|---|
| With aluminum tri-bromide In dichloromethane at 20℃; for 1h; Yield given. Further byproducts given. Yields of byproduct given; | |
| With aluminum tri-bromide In dichloromethane at 40℃; for 1.5h; Yield given. Further byproducts given. Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| With magnesium 1.) n-butyl ether, 2.) n-butyl ether, 70 deg C; Yield given. Multistep reaction; |
-
-
75-76-3
tetramethylsilane

-
-
935-56-8
1-chloroadamantane

-
A
-
702-79-4
1,3-dimethyladamantane

-
B
-
281-23-2
adamantane

-
-
768-91-2
1-methyl-adamantane

| Conditions | Yield |
|---|---|
| With aluminum tri-bromide In dichloromethane at 20℃; for 0.166667h; Further byproducts given; | A 37 % Chromat. B 12 % Chromat. C 38 % Chromat. |
| With aluminum tri-bromide In dichloromethane at 20℃; for 1h; Further byproducts given; | A 35 % Chromat. B 12 % Chromat. C 34 % Chromat. |

-
-
75-76-3
tetramethylsilane

-
-
935-56-8
1-chloroadamantane

-
A
-
702-79-4
1,3-dimethyladamantane

-
B
-
281-23-2
adamantane

-
C
-
1687-36-1
1,3,5,7-tetramethyladamantane

-
D
-
707-35-7
1,3,5-trimethyladamantane

-
-
768-91-2
1-methyl-adamantane

| Conditions | Yield |
|---|---|
| With aluminum tri-bromide In dichloromethane at 20℃; for 0.166667h; Product distribution; other time; various ratios of the educts and AlBr3; |

-
-
75-76-3
tetramethylsilane

-
-
935-56-8
1-chloroadamantane

-
A
-
702-79-4
1,3-dimethyladamantane

-
B
-
1687-36-1
1,3,5,7-tetramethyladamantane

-
C
-
707-35-7
1,3,5-trimethyladamantane

-
-
768-91-2
1-methyl-adamantane

| Conditions | Yield |
|---|---|
| With aluminum tri-bromide In dichloromethane at 20℃; for 0.166667h; Yield given. Further byproducts given. Yields of byproduct given; | |
| With aluminum tri-bromide In dichloromethane at 20℃; for 1h; Yield given. Further byproducts given. Yields of byproduct given; |

-
-
75-76-3
tetramethylsilane

-
-
281-23-2
adamantane

-
A
-
702-79-4
1,3-dimethyladamantane

-
-
768-91-2
1-methyl-adamantane

| Conditions | Yield |
|---|---|
| With aluminum tri-bromide In dichloromethane at 20℃; for 3h; Yield given. Yields of byproduct given; |

-
-
75-76-3
tetramethylsilane

-
-
281-23-2
adamantane

-
A
-
702-79-4
1,3-dimethyladamantane

-
B
-
1687-36-1
1,3,5,7-tetramethyladamantane

-
C
-
707-35-7
1,3,5-trimethyladamantane

-
-
768-91-2
1-methyl-adamantane

-
E
-
2109-06-0
1,3,5-Trimethyl-7-ethyladamantane

-
F
-
30904-21-3
1,2,3,5,7-pentamethyladamantane

| Conditions | Yield |
|---|---|
| With aluminum tri-bromide In dichloromethane at 20℃; for 6h; Product distribution; other time; other temperature; AlCl3 instead AlBr3; |

-
-
75-76-3
tetramethylsilane

-
-
281-23-2
adamantane

-
A
-
702-79-4
1,3-dimethyladamantane

-
B
-
707-35-7
1,3,5-trimethyladamantane

-
-
768-91-2
1-methyl-adamantane

| Conditions | Yield |
|---|---|
| With aluminum tri-bromide In dichloromethane at 20℃; for 6h; Yield given. Yields of byproduct given; |

-
-
702-79-4
1,3-dimethyladamantane

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride; phosphorus trichloride for 5h; Inert atmosphere; Reflux; | 99% |
-
-
702-79-4
1,3-dimethyladamantane

-
-
77287-34-4, 77287-35-5, 60100-09-6
formamide

-
-
351329-88-9
1-formamido-3,5-dimethyltricyclo[3.3.1.13,7]decane

| Conditions | Yield |
|---|---|
| Stage #1: 1,3-dimethyladamantane With sulfuric acid; nitric acid at 20 - 25℃; for 6.5h; Stage #2: formamide at 30 - 35℃; for 3h; | 96.96% |
| Stage #1: 1,3-dimethyladamantane With sulfuric acid; nitric acid at 0 - 5℃; Stage #2: formamide at 55 - 60℃; for 2h; Stage #3: With ammonia In dichloromethane; water at 0 - 25℃; | 95% |
| Stage #1: 1,3-dimethyladamantane With sulfuric acid; nitric acid at 0℃; for 6h; Stage #2: formamide at 0 - 20℃; for 2.5h; Ritter-type reaction; Further stages.; | 89% |
| Stage #1: 1,3-dimethyladamantane With sulfuric acid; nitric acid at 0℃; Stage #2: formamide at 0 - 20℃; | 89.3% |
| Stage #1: 1,3-dimethyladamantane With sulfuric acid; nitric acid at 0℃; Stage #2: formamide at 0 - 20℃; for 2h; |

| Conditions | Yield |
|---|---|
| Stage #1: 1,3-dimethyladamantane; trifluoroacetic acid With oxygen; sodium nitrite at 20℃; for 3h; Stage #2: With hydrogenchloride Further stages.; | 96% |

| Conditions | Yield |
|---|---|
| In benzene at 23℃; Kinetics; | 96% |

| Conditions | Yield |
|---|---|
| With water; bromine at 25℃; Reflux; | 95% |
| With oxygen; cobalt acetylacetonate In acetic acid at 80℃; under 760.051 Torr; for 15h; | 90% |
| With water; bromine for 2h; Ambient temperature; | 87% |
-
-
702-79-4
1,3-dimethyladamantane

-
-
131211-29-5
bis(3,5-dimethyl-1-adamantyl)phosphinic chloride

| Conditions | Yield |
|---|---|
| With aluminium trichloride; phosphorus trichloride at 75 - 85℃; for 5h; | 94.7% |
-
-
816444-61-8
N-[(1S)-amino(4-tolyl)oxido-λ4-sulfanylidene]tosylamide

-
-
702-79-4
1,3-dimethyladamantane

-
-
1396022-02-8
(S)-N-[[(3,5-dimethyl-1-adamantyl)amino](4-tolyl)oxido-λ4-sulfanylidene]tosylamide

| Conditions | Yield |
|---|---|
| With dirhodium tetrakis{(2S)-2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoic acid}; bis(tertbutylcarbonyloxy)iodobenzene In methanol; 1,2-dichloro-ethane at -35℃; for 72h; Molecular sieve; diastereoselective reaction; | 94% |

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride; bromine In 1,2-dichloro-ethane at 15℃; Reagent/catalyst; Temperature; Solvent; | 92% |
| With bromine for 12h; Reflux; | 91.5% |
| With iron(III)-acetylacetonate; carbon tetrabromide at 150℃; for 3h; Sealed tube; Inert atmosphere; | 85% |
-
-
24517-64-4
2-amino-3-pyridinecarbonitrile

-
-
702-79-4
1,3-dimethyladamantane

-
-
1514005-20-9
2-(((1r,3R,5S,7r)-3,5-dimethyladamantan-1-yl)amino)nicotinonitrile

| Conditions | Yield |
|---|---|
| With aluminum tri-bromide; carbon tetrabromide In neat (no solvent) at 15 - 20℃; for 0.0833333h; Green chemistry; chemoselective reaction; | 92% |
-
-
702-79-4
1,3-dimethyladamantane

-
-
75-05-8
acetonitrile

-
-
14931-70-5
1,3‑diacetamido‑5,7‑dimethyladamantane

| Conditions | Yield |
|---|---|
| Stage #1: 1,3-dimethyladamantane With nitric acid at 25℃; for 1h; Stage #2: acetonitrile for 0.5h; Stage #3: acetonitrile With sulfuric acid at 20℃; for 4h; Ritter Amidation; | 92% |
-
-
421-85-2
Trifluoromethanesulfonamide

-
-
702-79-4
1,3-dimethyladamantane

-
-
1309688-17-2
N-((1,3,5,7)-3,5-dimethyladamantan-1-yl)-1,1,1-trifluoromethanesulfonamide

| Conditions | Yield |
|---|---|
| With bis(4-bromobenzoyloxy)iodobenzene; iodine In dichloromethane at 20℃; for 14h; Schlenk technique; Inert atmosphere; Irradiation; | 92% |
-
-
67-56-1
methanol

-
-
702-79-4
1,3-dimethyladamantane

-
-
201230-82-2
carbon monoxide

-
-
24556-26-1
dimethyl 5,7-dimethyladamantane-1,3-dicarboxylate

| Conditions | Yield |
|---|---|
| Stage #1: 1,3-dimethyladamantane; carbon monoxide With Al2Br7(1-)*CBr3(1+) In 1,2-dibromomethane at 0℃; under 760.051 Torr; for 3h; Stage #2: methanol In 1,2-dibromomethane at 0 - 20℃; chemoselective reaction; | 91% |

-
-
702-79-4
1,3-dimethyladamantane

-
-
101821-77-6
3,5-dimethyladamantan-1-yl nitrate

| Conditions | Yield |
|---|---|
| With nitric acid at 10℃; | 90.3% |
| With nitric acid In acetic acid at 20℃; for 1h; | |
| With nitric acid for 3h; Cooling; | |
| With nitric acid In acetic acid at 15 - 20℃; for 1h; |

| Conditions | Yield |
|---|---|
| Stage #1: 1,3-dimethyladamantane With sulfuric acid; nitric acid at 0℃; for 6h; Stage #2: acetonitrile at 0 - 20℃; for 3.5h; Ritter-type reaction; Further stages.; | 90.3% |
| With sulfuric acid In tert-butyl alcohol at 60 - 65℃; for 18h; Ritter Amidation; Large scale; | 90% |
| With sulfuric acid at 60 - 80℃; for 10h; Ritter Amidation; | 73.5% |
-
-
702-79-4
1,3-dimethyladamantane

-
-
21912-23-2
1,3‑dibromo‑5,7‑dimethyladamantane

| Conditions | Yield |
|---|---|
| With bromine; iron In 1,2-dichloro-ethane at 25℃; Temperature; Reagent/catalyst; Solvent; | 90% |
| With bromine; iron at 20℃; for 1.5h; Bromination; | 83% |
-
-
64-18-6
formic acid

-
-
702-79-4
1,3-dimethyladamantane

-
-
13928-68-2
5,7-dimethyl-1,3-adamantanedicarboxylic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid at 20 - 28℃; for 5h; | 90% |
-
-
95-14-7
1,2,3-Benzotriazole

-
-
702-79-4
1,3-dimethyladamantane

-
-
1528767-79-4
1-((1r,3R,5S,7r)-3,5-dimethyladamantan-1-yl)-1H-benzo[d][1,2,3]triazole

| Conditions | Yield |
|---|---|
| With aluminum tri-bromide; carbon tetrabromide In neat (no solvent) at 15 - 20℃; for 0.0833333h; Green chemistry; chemoselective reaction; | 89% |
-
-
702-79-4
1,3-dimethyladamantane

-
-
1885-29-6
anthranilic acid nitrile

-
-
1521610-22-9
2-(((1r,3R,5S,7r)-3,5-dimethyladamantan-1-yl)amino)benzonitrile

| Conditions | Yield |
|---|---|
| With aluminum tri-bromide; carbon tetrabromide In neat (no solvent) at 15 - 20℃; for 0.0833333h; Green chemistry; chemoselective reaction; | 87% |
-
-
108-31-6
maleic anhydride

-
-
702-79-4
1,3-dimethyladamantane

-
-
119347-82-9
3-(3,5-Dimethyl-adamantan-1-yl)-dihydro-furan-2,5-dione

| Conditions | Yield |
|---|---|
| With di-tert-butyl peroxide In chlorobenzene at 150℃; for 5h; | 86% |
-
-
702-79-4
1,3-dimethyladamantane

-
-
60389-56-2
1,3-difluoro-5,7-dimethyladamantane

| Conditions | Yield |
|---|---|
| With iodine pentafluoride In 1,2-dichloro-ethane at 80℃; for 12h; regioselective reaction; | 86% |
-
-
702-79-4
1,3-dimethyladamantane

-
-
51-79-6
urethane

-
-
118648-19-4
1-ethoxycarbonylamino-3,5-dimethyladamantane

| Conditions | Yield |
|---|---|
| With nitric acid at 30℃; for 1h; | 85% |
| With nitric acid 1.) 25 deg C, 15 min, 2.) RT, 1 h; Yield given. Multistep reaction; |
-
-
702-79-4
1,3-dimethyladamantane

-
-
30934-81-7
1-fluoro-3,5-dimethyladamantane

| Conditions | Yield |
|---|---|
| With iodine pentafluoride In dichloromethane at 10℃; for 12h; regioselective reaction; | 85% |

| Conditions | Yield |
|---|---|
| With bis{rhodium[3,3'-(1,3-phenylene)bis(2,2-dimethylpropanoic acid)]}; [bis(acetoxy)iodo]benzene; sodium hydrogencarbonate for 2h; | 85% |
-
-
816444-61-8
N-[(1S)-amino(4-tolyl)oxido-λ4-sulfanylidene]tosylamide

-
-
702-79-4
1,3-dimethyladamantane

| Conditions | Yield |
|---|---|
| With Rh2(S-N-(1,8-naphthoyl)alanine)4; bis(tertbutylcarbonyloxy)iodobenzene In methanol; 1,1,2,2-tetrachloroethane at -78 - -35℃; for 72h; Molecular sieve; Inert atmosphere; | 83% |

| Conditions | Yield |
|---|---|
| With bis{rhodium[3,3'-(1,3-phenylene)bis(2,2-dimethylpropanoic acid)]}; [bis(acetoxy)iodo]benzene; sodium hydrogencarbonate for 2.5h; | 81% |

| Conditions | Yield |
|---|---|
| With bis{rhodium[3,3'-(1,3-phenylene)bis(2,2-dimethylpropanoic acid)]}; [bis(acetoxy)iodo]benzene; sodium hydrogencarbonate for 2.5h; | 80% |
1,3-Dimethyladamantane Chemical Properties
Molecular Structure: 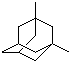
Molecular Formula: C12H20
Molecular Weight: 164.29
CAS NO: 702-79-4
EINECS: 211-870-8
H bond acceptors: 0
H bond donors: 0
Freely Rotating Bonds: 0
Polar Surface Area: 0 Å2
Index of Refraction: 1.52
Molar Refractivity: 51.44 cm3
Molar Volume: 168.9 cm3
Surface Tension: 39.1 dyne/cm
Density: 0.972 g/cm3
Flash Point: 52.8 °C
Enthalpy of Vaporization: 41.83 kJ/mol
Boiling Point: 199.9 °C at 760 mmHg
Vapour Pressure: 0.472 mmHg at 25°C
Melting Point: -30 °C
Stability: Stable. Incompatible with strong oxidizing agents. Flammable
Appearance: clear colourless liquid
IUPAC Name: 1,3-Dimethyladamantane
The chemical synonyms of 1,3-Dimethyladamantane (CAS NO.702-79-4): 1,3-Dimethyltricyclo(3.3.1.13,7)decane
Product Categories: Chemical intermediate for Memantine HCl;Adamantane derivatives;Adamantanes;Alkanes;Cyclic;Organic Building Blocks
1,3-Dimethyladamantane Safety Profile
1,3-Dimethyladamantane (CAS NO.702-79-4):
Hazard Codes:  F
F
Risk Statements: 10
R10:Flammable.
Safety Statements: 16
S16:Keep away from sources of ignition.
RIDADR UN: 1993 3/PG 3
WGK Germany: 3
HazardClass: 3
PackingGroup: III
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 70280-03-4
- 70280-88-5
- 702-81-8
- 702-82-9
- 70283-74-8
- 7028-48-0
- 70285-40-4
- 7028-67-3
- 70288-65-2
- 70288-86-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View