-
Name
1,4-Cyclohexanedimethanol
- EINECS 203-268-9
- CAS No. 105-08-8
- Article Data52
- CAS DataBase
- Density 1.004 g/cm3
- Solubility soluble in water
- Melting Point 31.5 °C
- Formula C8H16O2
- Boiling Point 286.2 °C at 760 mmHg
- Molecular Weight 144.214
- Flash Point 161.1 °C
- Transport Information
- Appearance clear colourless viscous liquid
- Safety 22-24/25-39-26
- Risk Codes 36
-
Molecular Structure
- Hazard Symbols R36:;
- Synonyms 1,4-Bis(hydroxymethyl)cyclohexane;1,4-Cyclohexamethylenebis methylol;1,4-Dimethylolcyclohexane;CHDM;CHDM-D;Cyclohex-1,4-ylenedimethanol;NSC 44508;Rikabinol DM;Sky CHDM;Vibracure A240;1,4-Cyclohexane dimethanol;
- PSA 40.46000
- LogP 0.77740
Synthetic route
-
-
619-81-8, 619-82-9, 1076-97-7
cyclohexane-1,4-dicarboxylic acid

-
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

| Conditions | Yield |
|---|---|
| With tin; hydrogen at 220℃; under 90009 Torr; for 0.75h; Temperature; Pressure; | 100% |
| With hydrogen In water at 230℃; under 15001.5 - 75007.5 Torr; Catalytic behavior; Reagent/catalyst; Autoclave; | 98.3% |
| With hydrogen In water at 230℃; under 63756.4 Torr; for 3h; Reagent/catalyst; Autoclave; | 95.8% |

| Conditions | Yield |
|---|---|
| With hydrogen In methanol at 200 - 240℃; under 30003 - 60006 Torr; for 8h; | 92% |
| With Cat.7; hydrogen at 180 - 250℃; under 52505.3 - 67506.8 Torr; Reagent/catalyst; Temperature; Pressure; |

| Conditions | Yield |
|---|---|
| With Ru/Al2O3; hydrogen In tetrahydrofuran at 60℃; under 7.50075 Torr; for 3.5h; Pressure; Autoclave; | 92% |

| Conditions | Yield |
|---|---|
| Stage #1: Bis(2-Hydroxyethyl)terephthalat With tin(II) chloride dihdyrate; ruthenium(III) chloride trihydrate; alumina; Cl6H6Pt(2-)*2H(1+)*6H2O; hydrogen In water at 180 - 260℃; under 37503.8 Torr; for 10h; Autoclave; Stage #2: With sodium tetrahydroborate; sodium hydroxide at 500℃; for 5.5h; pH=9; Reagent/catalyst; Concentration; Temperature; | 87.1% |

| Conditions | Yield |
|---|---|
| With C33H29FeMnN2O3P(1+)*Br(1-); potassium tert-butylate; hydrogen In isopropyl alcohol at 75℃; under 37503.8 Torr; Autoclave; | 86% |
| Stage #1: dimethyl 1,4-cyclohexane dicarboxylate With lithium aluminium tetrahydride In tetrahydrofuran at 0 - 20℃; for 0.5h; Stage #2: With water In tetrahydrofuran | |
| With hydrogen In methanol at 220℃; under 45004.5 Torr; Catalytic behavior; Time; Temperature; Green chemistry; |
-
-
959-26-2
Bis(2-Hydroxyethyl)terephthalat

-
A
-
94-08-6
4-methylbenzoic acid ethyl ester

-
B
-
106-42-3
para-xylene

-
C
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

| Conditions | Yield |
|---|---|
| With hydrogen at 170 - 260℃; under 37503.8 Torr; for 8h; Autoclave; | A n/a B n/a C 78.4% |


-
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

| Conditions | Yield |
|---|---|
| With hydrogen In neat (no solvent) at 269.84℃; under 78757.9 Torr; for 10h; Reagent/catalyst; Pressure; Temperature; Autoclave; | 78% |

-
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

| Conditions | Yield |
|---|---|
| With hydrogen In water at 160℃; under 45004.5 Torr; for 12h; Reagent/catalyst; | 56% |
-
-
959-26-2
Bis(2-Hydroxyethyl)terephthalat

-
A
-
94-08-6
4-methylbenzoic acid ethyl ester

-
B
-
106-42-3
para-xylene

-
C
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

| Conditions | Yield |
|---|---|
| With hydrogen at 230℃; under 37503.8 Torr; for 8h; Autoclave; | A n/a B n/a C 47.2% D n/a E n/a |

-
-
959-26-2
Bis(2-Hydroxyethyl)terephthalat

-
A
-
94-08-6
4-methylbenzoic acid ethyl ester

-
B
-
106-42-3
para-xylene

-
C
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

-
D
-
34885-03-5
(4-methylcyclohexyl)methanol

-
E
-
589-90-2
1,4 dimethylcyclohexane

| Conditions | Yield |
|---|---|
| With hydrogen at 260℃; under 37503.8 Torr; for 8h; Autoclave; | A n/a B n/a C 28.5% D n/a E n/a |

-
-
959-26-2
Bis(2-Hydroxyethyl)terephthalat

-
A
-
94-08-6
4-methylbenzoic acid ethyl ester

-
B
-
106-42-3
para-xylene

-
C
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

-
E
-
34885-03-5
(4-methylcyclohexyl)methanol

-
F
-
589-90-2
1,4 dimethylcyclohexane

| Conditions | Yield |
|---|---|
| With hydrogen at 170 - 260℃; under 37503.8 Torr; for 8h; Autoclave; | A n/a B n/a C 14.7% D n/a E n/a F n/a |

-
-
959-26-2
Bis(2-Hydroxyethyl)terephthalat

-
A
-
94-08-6
4-methylbenzoic acid ethyl ester

-
B
-
106-42-3
para-xylene

-
C
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

| Conditions | Yield |
|---|---|
| With hydrogen at 170 - 260℃; under 37503.8 Torr; for 8h; Autoclave; | A n/a B n/a C 5.3% D n/a |

-
-
120-61-6
1,4-benzenedicarboxylic acid dimethyl ester

-
A
-
94-60-0
dimethyl 1,4-cyclohexane dicarboxylate

-
B
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

-
C
-
62172-89-8
4-methyloxymethylhydroxymethylhydroxymethylcyclohexane

| Conditions | Yield |
|---|---|
| With hydrogen; Pt1Ru5; silica gel In ethanol at 99.85℃; under 15001.2 Torr; for 8h; Product distribution; Further Variations:; Catalysts; reaction times; |

-
-
120-61-6
1,4-benzenedicarboxylic acid dimethyl ester

-
A
-
94-60-0
dimethyl 1,4-cyclohexane dicarboxylate

-
B
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

| Conditions | Yield |
|---|---|
| With hydrogen; Ru5PtSn; silica gel In ethanol at 99.85℃; under 15001.2 Torr; for 24h; |
-
-
120-61-6
1,4-benzenedicarboxylic acid dimethyl ester

-
A
-
94-60-0
dimethyl 1,4-cyclohexane dicarboxylate

-
B
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

-
C
-
51181-40-9
methyl 4-methyl-1-cyclohexanecarboxylate

-
D
-
34885-03-5
(4-methylcyclohexyl)methanol

| Conditions | Yield |
|---|---|
| With hydrogen; Ru5PtSn; silica gel In ethanol at 119.85℃; under 30002.4 Torr; for 8h; Further byproducts given; | |
| With hydrogen; Ru5PtSn; silica gel In ethanol at 119.85℃; under 15001.2 Torr; for 24h; Further byproducts given; |

-
-
120-61-6
1,4-benzenedicarboxylic acid dimethyl ester

-
A
-
94-60-0
dimethyl 1,4-cyclohexane dicarboxylate

-
B
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

-
C
-
98955-27-2
[4-(methoxymethyl)cyclohexyl]methanol

| Conditions | Yield |
|---|---|
| With hydrogen; Ru5PtSn; silica gel In ethanol at 99.85℃; under 15001.2 Torr; for 24h; Product distribution; Further Variations:; Catalysts; Temperatures; Pressures; | |
| With hydrogen; Pt1Ru5; silica gel In ethanol at 99.85℃; under 15001.2 Torr; for 24h; |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 6 h / 260 - 280 °C / 4535.33 Torr 2: dimethyl 1,4-cyclohexane dicarboxylate; Pd/Al2O3; hydrogen / 1 h / 200 °C / 93849.2 Torr / Autoclave 3: (4-methylcyclohexyl)methanol; hydrogen / 5 h / 250 °C / 212036 Torr View Scheme | |
| Stage #1: terephthalic acid With palladium on activated charcoal; hydrogen In water at 230℃; under 60006 Torr; Stage #2: at 230℃; Pressure; Temperature; Reagent/catalyst; | 100 %Chromat. |
| With hydrogen In water at 20 - 230℃; under 60006 Torr; Catalytic behavior; Reagent/catalyst; Inert atmosphere; |
-
-
95623-88-4
terephthalic acid 1,4-bis(4-methylcyclohexyl)methyl ester

-
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: dimethyl 1,4-cyclohexane dicarboxylate; Pd/Al2O3; hydrogen / 1 h / 200 °C / 93849.2 Torr / Autoclave 2: (4-methylcyclohexyl)methanol; hydrogen / 5 h / 250 °C / 212036 Torr View Scheme | |
| With hydrogen at 150℃; under 22502.3 Torr; Temperature; Pressure; |
-
-
1420954-46-6
bis((4-methylcyclohexyl)methyl) cyclohexane-1,4-dicarboxylate

-
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

| Conditions | Yield |
|---|---|
| With (4-methylcyclohexyl)methanol; hydrogen at 250℃; under 212036 Torr; for 5h; |
-
-
94-60-0
dimethyl 1,4-cyclohexane dicarboxylate

-
A
-
13380-85-3
4-hydroxymethylcyclohexanecarboxylic acid methyl ester

-
B
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

| Conditions | Yield |
|---|---|
| With hydrogen In methanol at 220℃; under 45004.5 Torr; Green chemistry; | |
| With hydrogen In methanol at 219.84℃; under 37503.8 Torr; Reagent/catalyst; chemoselective reaction; | |
| With hydrogen In isopropyl alcohol at 220℃; under 75007.5 Torr; for 10h; Reagent/catalyst; Autoclave; |
-
-
94-60-0
dimethyl 1,4-cyclohexane dicarboxylate

-
A
-
13380-85-3
4-hydroxymethylcyclohexanecarboxylic acid methyl ester

-
B
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

-
C
-
34885-03-5
(4-methylcyclohexyl)methanol

-
D
-
92385-32-5
4-(hydroxymethyl)-1-cyclohexanecarboxaldehyde

| Conditions | Yield |
|---|---|
| With hydrogen at 220℃; under 45004.5 Torr; Catalytic behavior; Reagent/catalyst; Time; Overall yield = 98.7 %; |

-
-
94-60-0
dimethyl 1,4-cyclohexane dicarboxylate

-
A
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

-
B
-
34885-03-5
(4-methylcyclohexyl)methanol

-
C
-
92385-32-5
4-(hydroxymethyl)-1-cyclohexanecarboxaldehyde

| Conditions | Yield |
|---|---|
| With hydrogen at 220℃; under 45004.5 Torr; Catalytic behavior; Reagent/catalyst; Time; Overall yield = 67.4 %; |

-
-
94-60-0
dimethyl 1,4-cyclohexane dicarboxylate

-
A
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

-
B
-
33424-83-8
1,4-cyclohexanedicarboxaldehyde

| Conditions | Yield |
|---|---|
| With hydrogen at 180℃; under 30003 Torr; Temperature; Pressure; Concentration; |
-
-
94-60-0
dimethyl 1,4-cyclohexane dicarboxylate

-
A
-
13380-85-3
4-hydroxymethylcyclohexanecarboxylic acid methyl ester

-
B
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

-
C
-
34885-03-5
(4-methylcyclohexyl)methanol

| Conditions | Yield |
|---|---|
| With hydrogen In isopropyl alcohol at 220℃; under 75007.5 Torr; for 10h; Reagent/catalyst; Pressure; Autoclave; |

-
-
94-60-0
dimethyl 1,4-cyclohexane dicarboxylate

-
A
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

-
B
-
34885-03-5
(4-methylcyclohexyl)methanol

| Conditions | Yield |
|---|---|
| With hydrogen In isopropyl alcohol at 220℃; under 75007.5 Torr; for 10h; Reagent/catalyst; Pressure; Autoclave; |
-
-
61-90-5
L-leucine

-
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

-
-
893447-84-2
L,L-leucine 1,4-cyclohexanedimethanol diester

| Conditions | Yield |
|---|---|
| Stage #1: L-leucine; bis-1,4-(hydroxymethyl)cyclohexane With toluene-4-sulfonic acid In toluene at 150℃; for 30h; Heating / reflux; Stage #2: With sodium hydrogencarbonate In water; toluene at 60℃; | 100% |
-
-
61-90-5
L-leucine

-
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

-
-
893447-84-2
L,L-leucine 1,4-cyclohexanedimethanol diester

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene at 20 - 150℃; for 30h; Heating / reflux; | 100% |
-
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

-
-
108-24-7
acetic anhydride

-
-
6308-18-5
1,4-cyclohexanedimethylene diacetate

| Conditions | Yield |
|---|---|
| With pyridine at 125℃; for 3.5h; Reagent/catalyst; Large scale; | 99% |
| With pyridine at 125℃; for 3.5h; Large scale; | 99% |
-
-
5453-80-5, 19926-90-0
5-Norbornene-2-carboxaldehyde

-
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

| Conditions | Yield |
|---|---|
| sodium hydride at 20℃; for 10h; | 93% |
-
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

-
-
59274-95-2
7-oxa-bicyclo[2.2.1]hept-5-ene-2-carbaldehyde

| Conditions | Yield |
|---|---|
| sodium hydride at 20℃; for 10h; | 93% |

| Conditions | Yield |
|---|---|
| With Pt-Sn/γ-Al2O3 In o-xylene at 145℃; for 24h; Inert atmosphere; | 93% |

| Conditions | Yield |
|---|---|
| With pyridine; thionyl chloride Chlorination; Heating; | 92% |
-
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

-
-
1885-14-9
phenyl chloroformate

-
-
22563-70-8
1,4-cyclohexanedimethanol diphenyl dicarbonate

| Conditions | Yield |
|---|---|
| With pyridine; dmap In tetrahydrofuran at 0 - 20℃; | 90.2% |

| Conditions | Yield |
|---|---|
| With dibutyltin dilaurate In toluene for 5h; | 90.1% |
-
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

-
-
75-36-5
acetyl chloride

-
-
6308-18-5
1,4-cyclohexanedimethylene diacetate

| Conditions | Yield |
|---|---|
| With pyridine; dmap In chloroform at 0 - 20℃; | 87% |
-
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

-
-
60631-76-7
(R)-1-C-(1-Cyclohexen-5-yl)carbaldehyde

-
-
100-49-2
cyclohexylmethyl alcohol

| Conditions | Yield |
|---|---|
| With N,N-dimethyl acetamide; toluene-4-sulfonic acid at 140℃; for 20h; | 85% |

-
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

-
-
2524-64-3
chlorophosphoric acid diphenyl ester

-
-
476371-27-4
1,4-cyclohexanedimethanol bis(diphenyl phosphate)

| Conditions | Yield |
|---|---|
| With magnesium chloride In methanol; hexane | 82.2% |

| Conditions | Yield |
|---|---|
| With dmap; 2,6-di-tert-butyl-4-methyl-phenol; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 48h; | 81% |
-
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

-
-
10534-13-1
cyclohexane-1,4-dicarbonitrile

| Conditions | Yield |
|---|---|
| With 1,10-Phenanthroline; ammonia; oxygen In 1,4-dioxane at 30 - 60℃; under 3750.38 Torr; for 12h; Pressure; Temperature; Reagent/catalyst; Solvent; | 80.5% |
-
-
108-31-6
maleic anhydride

-
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

-
-
551929-43-2
(Z)-2-butenedioic-acid-1,4-cyclohexanediylbis(methylene) ester

| Conditions | Yield |
|---|---|
| In acetonitrile at 50℃; for 72h; | 80% |
| In acetonitrile at 50℃; for 72h; | 80% |
-
-
13380-84-2
4-(hydroxymethyl)cyclohexane-1-carboxylic acid

-
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

| Conditions | Yield |
|---|---|
| Stage #1: bis-1,4-(hydroxymethyl)cyclohexane With dmap; dicyclohexyl-carbodiimide In dimethyl sulfoxide at 20℃; for 0.5h; Stage #2: 4-(hydroxymethyl)cyclohexane-1-carboxylic acid In dimethyl sulfoxide at 20℃; for 5h; | 80% |
| Stage #1: bis-1,4-(hydroxymethyl)cyclohexane With dmap; dicyclohexyl-carbodiimide In dimethyl sulfoxide at 20℃; for 0.5h; Stage #2: 4-(hydroxymethyl)cyclohexane-1-carboxylic acid In dimethyl sulfoxide at 20℃; for 5h; | 80% |
-
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

| Conditions | Yield |
|---|---|
| With iron(III) chloride In 1,2-dichloro-ethane at 70℃; for 10h; | 78% |
-
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

-
-
1187856-44-5
(4-chlorophenyl)(phenyl)carbamic chloride

| Conditions | Yield |
|---|---|
| With pyridine at 120℃; for 18h; | 78% |
-
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

-
-
110844-29-6
1,5-dioxa-3,3-bis(ethoxycarbonyl)-8,8,10,10-tetramethyl-9-azaspiro<5.5>undecane

-
-
110839-53-7
8,8,10,10-Tetramethyl-1,5-dioxa-9-azaspiro-[5.5]undecane-3,3-dicarboxylic acid

| Conditions | Yield |
|---|---|
| In dichloromethane; acetic acid | 77% |
-
-
108-22-5
Isopropenyl acetate

-
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

-
A
-
6308-18-5
1,4-cyclohexanedimethylene diacetate

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)diiridium(I) dichloride; sodium carbonate In toluene at 100℃; for 6h; | A n/a B 75% C n/a |


| Conditions | Yield |
|---|---|
| With Ni/NiO-diatomite at 200℃; for 3h; Reagent/catalyst; Autoclave; Inert atmosphere; | 72.6% |
| Multi-step reaction with 2 steps 1: 52.1 percent / 140 - 165 °C 2: 46.3 percent / aq. NaOH / 10 h / Heating View Scheme |
-
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
109004-13-9
[4-(p-toluenesulfonyloxymethyl)cyclohexyl]methanol

| Conditions | Yield |
|---|---|
| With pyridine; dmap; triethylamine In dichloromethane at 20℃; for 3h; | 72% |
| In pyridine at 20℃; for 36h; | 61% |
| With pyridine In dichloromethane | 186 g |
-
-
105-08-8
bis-1,4-(hydroxymethyl)cyclohexane

-
-
89607-68-1
ethyl 3-diazoindole-2-carboxylate

| Conditions | Yield |
|---|---|
| dirhodium tetraacetate In dichloromethane at 85℃; for 3h; Substitution; O-H insertation; | 72% |
1,4-Cyclohexanedimethanol Consensus Reports
1,4-Cyclohexanedimethanol Specification
The 1,4-Bis(hydroxymethyl)cyclohexane is an organic compound with the formula C8H16O2. The IUPAC name of this chemical is [4-(hydroxymethyl)cyclohexyl]methanol. With the CAS registry number 105-08-8, it is also named as Cyclohexan-1,4-diyldimethanol. The product's category is Alkohols. Besides, it is clear colourless viscous liquid, which should be stored in a cool and well-ventilated place. It is used in the manufacture of polyester fiber, polyester electrical appliances, unsaturated polyester resin, polyester glaze, polyurethane foam.
Physical properties about 1,4-Bis(hydroxymethyl)cyclohexane are: (1)ACD/LogP: 0.36; (2)ACD/LogD (pH 5.5): 0.36; (3)ACD/LogD (pH 7.4): 0.36; (4)ACD/BCF (pH 5.5): 1.1; (5)ACD/BCF (pH 7.4): 1.1; (6)ACD/KOC (pH 5.5): 37.33; (7)ACD/KOC (pH 7.4): 37.33; (8)#H bond acceptors: 2; (9)H bond donors: 2; (10)#Freely Rotating Bonds: 4; (11)Polar Surface Area: 18.46 Å2; (12)Index of Refraction: 1.47; (13)Molar Refractivity: 40.12 cm3; (14)Molar Volume: 143.5 cm3; (15)Polarizability: 15.9×10-24cm3; (16)Surface Tension: 40.5 dyne/cm; (17)Density: 1.004 g/cm3; (18)Flash Point: 161.1 °C; (19)Enthalpy of Vaporization: 60.96 kJ/mol; (20)Boiling Point: 286.2 °C at 760 mmHg; (21)Vapour Pressure: 0.000303 mmHg at 25°C.
Preparation: this chemical can be prepared by trans-1,4-bis-acetoxymethyl-cyclohexane. This reaction will need reagent methanolic potassium carbonate at ambient temperature. The yield is about 100%.
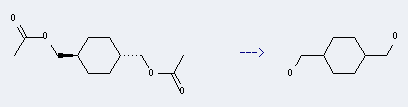
Uses of 1,4-Bis(hydroxymethyl)cyclohexane: it can be used to produce trans-1,4-Bis(chloromethyl)-cyclohexane. It will need reagent CCl4, Ph3P. The yield is about 64%.

When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes. When you are using it, wear suitable protective clothing, do not breathe dust and avoid contact with skin and eyes.
You can still convert the following datas into molecular structure:
(1)SMILES: OCC1CCC(CO)CC1
(2)InChI: InChI=1/C8H16O2/c9-5-7-1-2-8(6-10)4-3-7/h7-10H,1-6H2
(3)InChIKey: YIMQCDZDWXUDCA-UHFFFAOYAS
(4)Std. InChI: InChI=1S/C8H16O2/c9-5-7-1-2-8(6-10)4-3-7/h7-10H,1-6H2
(5)Std. InChIKey: YIMQCDZDWXUDCA-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | oral | 1600mg/kg (1600mg/kg) | Kodak Company Reports. Vol. 21MAY1971, | |
| mouse | LDLo | intraperitoneal | 1600mg/kg (1600mg/kg) | "Toxicology of Drugs and Chemicals," Deichmann, W.B., New York, Academic Press, Inc., 1969Vol. -, Pg. 194, 1969. | |
| rat | LD50 | oral | 3200mg/kg (3200mg/kg) | Kodak Company Reports. Vol. 21MAY1971, | |
| rat | LDLo | intraperitoneal | 800mg/kg (800mg/kg) | "Toxicology of Drugs and Chemicals," Deichmann, W.B., New York, Academic Press, Inc., 1969Vol. -, Pg. 194, 1969. |
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 105-09-9
- 105101-25-5
- 105102-20-3
- 105102-22-5
- 10510-54-0
- 105107-33-3
- 105107-84-4
- 105112-76-3
- 105-11-3
- 10511-51-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View