-
Name
1,8-Diiodonaphthalene
- EINECS
- CAS No. 1730-04-7
- Article Data22
- CAS DataBase
- Density 2.265 g/cm3
- Solubility
- Melting Point 109-113°C
- Formula C10H6I2
- Boiling Point 384.2 °C at 760 mmHg
- Molecular Weight 379.967
- Flash Point 204.3 °C
- Transport Information
- Appearance
- Safety 60-61
- Risk Codes 22-50/53
-
Molecular Structure
- Hazard Symbols Xn,N
- Synonyms Naphthalene,1,8-diiodo-;
- PSA 0.00000
- LogP 4.04900
Synthetic route

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene; iodine In tetrachloromethane for 2h; Heating; Irradiation; | 80% |

| Conditions | Yield |
|---|---|
| With sulfuric acid; 7,14-bis(trimethylsilyl)acenaphtho[1,2-k]fluoranthene; sodium nitrite In water at -20 - 80℃; for 0.5h; Inert atmosphere; | 70% |
| Stage #1: naphthalene-1,8-diamine With sulfuric acid; sodium nitrite In water at -20 - -15℃; Stage #2: With potassium iodide In water | 63% |
| Stage #1: naphthalene-1,8-diamine With sulfuric acid; sodium nitrite In water at -20 - -15℃; Stage #2: With potassium iodide In water at -20 - 80℃; | 61% |

| Conditions | Yield |
|---|---|
| With potassium phosphate; iodine In acetonitrile at 140℃; for 16h; Glovebox; Inert atmosphere; chemoselective reaction; | A 7% B 57% |

-
-
1730-04-7
1,8-diiodonaphthalene

| Conditions | Yield |
|---|---|
| (i) NaNO2, aq. HCl, (ii) KI; Multistep reaction; | |
| With hydrogenchloride; potassium iodide; sodium nitrite 1.) H2O, -1 deg C, 2.) H2O, heating, 2 h; Multistep reaction; |

-
-
1730-04-7
1,8-diiodonaphthalene

| Conditions | Yield |
|---|---|
| With hydrogenchloride Behandeln der Diazoniumsalzloesung mit KI; |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; diisopropylamine; tetrakis(triphenylphosphine) palladium(0) at 50℃; for 16h; Sonogashira reaction; | 99% |
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triethylamine; triphenylphosphine at 80℃; for 20h; Sonogashira coupling; Inert atmosphere; Sealed flask; | 94% |
-
-
1730-04-7
1,8-diiodonaphthalene

-
-
118486-94-5
2-(tributylstannyl)furan

-
-
1248481-21-1
1,8-di(furan-2-yl)naphthalene

| Conditions | Yield |
|---|---|
| With copper(l) iodide; tetrakis(triphenylphosphine) palladium(0); cesium fluoride In N,N-dimethyl-formamide at 45℃; for 14h; Inert atmosphere; | 99% |
-
-
1730-04-7
1,8-diiodonaphthalene

-
-
208399-66-0
4-methoxy-2-methylphenyl boronic acid

| Conditions | Yield |
|---|---|
| With potassium phosphate; tetrakis(triphenylphosphine) palladium(0) In toluene at 100℃; for 18h; Suzuki coupling; Inert atmosphere; | 99% |
| With potassium phosphate; tetrakis(triphenylphosphine) palladium(0) In toluene at 100℃; for 18h; Product distribution / selectivity; | 99% |

-
-
1730-04-7
1,8-diiodonaphthalene

-
-
81698-99-9
1,3,5-trimethyl 2-(α-furyl)benzene

| Conditions | Yield |
|---|---|
| Stage #1: 1,3,5-trimethyl 2-(α-furyl)benzene With n-butyllithium In tetrahydrofuran; hexane at 0℃; for 0.5h; Inert atmosphere; Stage #2: indium(III) chloride In tetrahydrofuran at -78 - 25℃; for 0.5h; Stage #3: 1,8-diiodonaphthalene With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride In tetrahydrofuran; methanol for 18h; Reflux; | 99% |

-
-
1730-04-7
1,8-diiodonaphthalene

-
-
116487-12-8
(4-Ethynyl-furan-3-yl)-trimethyl-silane

-
-
216309-10-3
1,8-bis-[(4-trimethylsilyl)-3-furanylethynyl]naphthalene

| Conditions | Yield |
|---|---|
| With triethylamine; bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide at 25℃; for 48h; | 97% |
-
-
14275-42-4
di-n-butylchlorogermane

-
-
1730-04-7
1,8-diiodonaphthalene

-
-
1578224-45-9
1,8-bis(di-n-butylgermyl)naphthalene

| Conditions | Yield |
|---|---|
| With n-butyllithium In diethyl ether; hexane at -60℃; for 1.5h; Inert atmosphere; | 96% |
-
-
1730-04-7
1,8-diiodonaphthalene

-
-
1066-54-2
trimethylsilylacetylene

-
-
27503-44-2
1,8-bis(2-trimethylsilylethynyl)naphthalene

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triethylamine at 20℃; for 12h; Sonogashira Cross-Coupling; Inert atmosphere; | 95% |
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triethylamine at 100℃; for 1h; Substitution; Sonogashira coupling; | 91% |
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triethylamine at 110℃; for 1h; Schlenk technique; Inert atmosphere; | 80% |
| Stage #1: trimethylsilylacetylene With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 0.5h; Stage #2: With zinc(II) chloride In tetrahydrofuran; hexane at 20℃; for 1h; Stage #3: 1,8-diiodonaphthalene With tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran; hexane at 20 - 50℃; Negishi coupling; Inert atmosphere; | 63% |
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; diethylamine | 30% |
-
-
1730-04-7
1,8-diiodonaphthalene

-
-
17356-19-3
1-ethynylcyclopentanol

-
-
1321605-56-4
1,1'-(naphthalene-1,8-diylbis(ethyne-2,1-diyl))dicyclopentanol

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triethylamine; triphenylphosphine at 80℃; for 20h; Sonogashira coupling; Inert atmosphere; Sealed flask; | 95% |
-
-
5419-55-6
Triisopropyl borate

-
-
1730-04-7
1,8-diiodonaphthalene

-
-
1400770-57-1
diisopropyl 8-iodonaphthalen-1-ylboronate

| Conditions | Yield |
|---|---|
| Stage #1: 1,8-diiodonaphthalene With isopropylmagnesium chloride In tetrahydrofuran; diethyl ether at -78℃; for 2h; Inert atmosphere; Stage #2: Triisopropyl borate In tetrahydrofuran; diethyl ether at -78 - 25℃; Inert atmosphere; regioselective reaction; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: furan With n-butyllithium In tetrahydrofuran; hexane at 0℃; for 0.5h; Inert atmosphere; Stage #2: indium(III) chloride In tetrahydrofuran at -78 - 25℃; for 0.5h; Stage #3: 1,8-diiodonaphthalene With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride In tetrahydrofuran; methanol for 18h; Reagent/catalyst; Concentration; Negishi Coupling; Reflux; | 95% |

-
-
498-66-8
norborn-2-ene

-
-
1730-04-7
1,8-diiodonaphthalene

-
-
57704-85-5, 75196-53-1, 119945-30-1
(6bα,7β,10β,10aα)-6b,7,8,9,10,10a-hexahydro-7,10-methanofluoranthene

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; potassium carbonate; palladium diacetate In N,N-dimethyl-formamide at 100℃; for 72h; | 94% |

| Conditions | Yield |
|---|---|
| With 2,4,6-trimethyl-pyridine; copper(II) oxide for 10h; Reflux; Inert atmosphere; | 94% |
| With copper(I) oxide In pyridine for 2h; Heating; | 55% |
-
-
1730-04-7
1,8-diiodonaphthalene

-
-
115-19-5
2-methyl-but-3-yn-2-ol

-
A
-
205124-36-3
4-(8-iodonaphthalene-1-yl)-2-methylbut-3-yn-2-ol

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triethylamine; triphenylphosphine at 20 - 80℃; Sonogashira coupling reaction; Inert atmosphere; Sealed flask; | A 5% B 94% |


| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triethylamine; triphenylphosphine at 80℃; for 20h; Sonogashira coupling; Inert atmosphere; Sealed flask; | 93% |
| With copper(l) iodide; bis(triphenylphosphine)palladium(II)-chloride; triethylamine; triphenylphosphine at 20 - 50℃; for 10h; Sonogashira coupling; Inert atmosphere; | 93% |
| Stage #1: 1,8-diiodonaphthalene With copper(l) iodide; tetrakis(triphenylphosphine) palladium(0); triethylamine; triphenylphosphine at 20℃; for 0.166667h; Sealed tube; Stage #2: 4-methoxyphenylacetylen at 20℃; for 16h; Sealed tube; | 42% |
-
-
1730-04-7
1,8-diiodonaphthalene

-
-
77-75-8
meparfynol

-
-
1321605-58-6
1,1'-(naphthalene-1,8-diyl)bis(3-methylpent-1-yn-3-ol)

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triethylamine; triphenylphosphine at 80℃; for 20h; Sonogashira coupling; Inert atmosphere; Sealed flask; | 93% |
-
-
1730-04-7
1,8-diiodonaphthalene

-
-
5720-06-9
2-Methoxyphenylboronic acid

-
-
1257841-02-3
1,8-bis(2-methoxyphenyl)naphthalene

| Conditions | Yield |
|---|---|
| With dicyclohexyl-(2',6'-dimethoxybiphenyl-2-yl)-phosphane; tris-(dibenzylideneacetone)dipalladium(0); caesium carbonate In water; N,N-dimethyl-formamide at 125℃; for 2h; Suzuki-Miyaura Coupling; Inert atmosphere; | 93% |
-
-
1730-04-7
1,8-diiodonaphthalene

-
-
115-19-5
2-methyl-but-3-yn-2-ol

-
-
205124-36-3
4-(8-iodonaphthalene-1-yl)-2-methylbut-3-yn-2-ol

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triethylamine; triphenylphosphine Sonogashira coupling reaction; | 92% |
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triethylamine; triphenylphosphine at 80℃; for 20h; Sonogashira coupling; Inert atmosphere; Sealed flask; | 92% |
| With triethylamine; copper(l) iodide; Pd2(dba)4; triphenylphosphine In toluene at 23℃; for 12h; Sonogashira reaction; | 83% |
-
-
13331-23-2
fur-2-ylboronic acid

-
-
1730-04-7
1,8-diiodonaphthalene

-
-
1248481-21-1
1,8-di(furan-2-yl)naphthalene

| Conditions | Yield |
|---|---|
| With copper(l) iodide; tetrakis(triphenylphosphine) palladium(0); cesium fluoride Inert atmosphere; | 92% |
-
-
6285-06-9
3-ethyl-1-pentyn-3-ol

-
-
1730-04-7
1,8-diiodonaphthalene

-
-
1321605-57-5
1,1'-(naphthalene-1,8-diyl)bis(3-ethylpent-1-yn-3-ol)

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triethylamine; triphenylphosphine at 80℃; for 20h; Sonogashira coupling; Inert atmosphere; Sealed flask; | 92% |
-
-
1730-04-7
1,8-diiodonaphthalene

-
-
766-97-2
4-n-methylphenylacetylene

-
-
17873-54-0
1,8-bis((p-tolyl)ethynyl)naphthalene

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triethylamine; triphenylphosphine at 80℃; for 20h; Sonogashira coupling; Inert atmosphere; Sealed flask; | 92% |
| With copper(l) iodide; bis(triphenylphosphine)palladium(II)-chloride; triethylamine; triphenylphosphine at 20 - 50℃; for 10h; Sonogashira coupling; Inert atmosphere; | 92% |
-
-
1730-04-7
1,8-diiodonaphthalene

-
-
766-46-1
2-bromo-1-ethynylbenzene

-
-
867194-03-4
1,8-bis[(2-bromophenyl)ethynyl]naphthalene

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triphenylphosphine In triethylamine at 50℃; for 12h; Sonogashira coupling; | 91% |
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triethylamine at 40 - 50℃; Inert atmosphere; Schlenk technique; Glovebox; |
-
-
1730-04-7
1,8-diiodonaphthalene

-
-
536-74-3
phenylacetylene

-
-
17694-87-0
1,8-bis-(phenylethynyl)naphthalene

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triethylamine; triphenylphosphine at 80℃; for 20h; Sonogashira coupling; Inert atmosphere; Sealed flask; | 90% |
| With copper(l) iodide; bis(triphenylphosphine)palladium(II)-chloride; triethylamine; triphenylphosphine at 20 - 50℃; for 10h; Sonogashira coupling; Inert atmosphere; | 90% |
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triphenylphosphine In triethylamine at 40℃; for 11h; Sonogashira coupling; | 89% |
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triethylamine In tetrahydrofuran at 20℃; for 12h; Sonogashira coupling; Inert atmosphere; | 86% |
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triethylamine at 20℃; Sonogashira Cross-Coupling; | 86% |
-
-
1730-04-7
1,8-diiodonaphthalene

-
-
101251-09-6
(4-acetylaminophenyl)boronic acid

-
-
1246218-84-7
1,8-bis(4'-acetamidophenyl)naphthalene

| Conditions | Yield |
|---|---|
| With potassium phosphate; tetrakis(triphenylphosphine) palladium(0) In ethanol; water; toluene at 95℃; for 20h; Suzuki coupling; Inert atmosphere; | 90% |
-
-
4339-05-3
3-methylhex-1-yn-3-ol

-
-
1730-04-7
1,8-diiodonaphthalene

-
-
1321605-59-7
1,1'-(naphthalene-1,8-diyl)bis(3-methylhex-1-yn-3-ol)

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triethylamine; triphenylphosphine at 80℃; for 20h; Sonogashira coupling; Inert atmosphere; Sealed flask; | 90% |
-
-
1730-04-7
1,8-diiodonaphthalene

-
-
78-27-3
1-Ethynyl-1-cyclohexanol

-
-
1321605-55-3
1,1'-(naphthalene-1,8-diylbis(ethyne-2,1-diyl))dicyclohexanol

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triethylamine; triphenylphosphine at 80℃; for 20h; Sonogashira coupling; Inert atmosphere; Sealed flask; | 90% |

| Conditions | Yield |
|---|---|
| Stage #1: 1,8-diiodonaphthalene With n-butyllithium In diethyl ether; hexane at -40℃; for 1h; Inert atmosphere; Stage #2: With 1,2-dibromo-1,1,2,2-tetrachloroethane In diethyl ether at -40 - 20℃; Inert atmosphere; | 90% |
-
-
1730-04-7
1,8-diiodonaphthalene

-
-
34837-55-3
Phenylselenyl bromide

-
-
1111098-36-2
1,8-bis(phenylselanyl)naphthalene

| Conditions | Yield |
|---|---|
| Stage #1: 1,8-diiodonaphthalene With n-butyllithium In tetrahydrofuran at -78℃; for 0.333333h; Inert atmosphere; Stage #2: Phenylselenyl bromide In tetrahydrofuran at -78 - 20℃; | 89% |
-
-
1730-04-7
1,8-diiodonaphthalene

-
-
1255533-04-0
1-(2,6-dichlorophenylethynyl)-8-iodonaphthalene

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triethylamine; triphenylphosphine at 20 - 50℃; Sonogashira coupling; Inert atmosphere; | 88% |
-
-
110-91-8
morpholine

-
-
1730-04-7
1,8-diiodonaphthalene

-
-
201230-82-2
carbon monoxide

-
-
1006863-06-4
1,8-bis[N,N-(3'-oxapenta-1',5'-diyl)carboxamido]-naphthalene

| Conditions | Yield |
|---|---|
| With triethylamine; triphenylphosphine; palladium diacetate In N,N-dimethyl-formamide at 50℃; under 750.075 Torr; for 66h; | 87% |

-
-
1730-04-7
1,8-diiodonaphthalene

-
-
1111098-40-8
1,8-bis[(p-tert-butylphenyl)selanyl]naphthalene

| Conditions | Yield |
|---|---|
| Stage #1: 1,8-diiodonaphthalene With n-butyllithium In tetrahydrofuran at -78℃; for 0.333333h; Inert atmosphere; Stage #2: C10H13BrSe In tetrahydrofuran at -78 - 20℃; | 87% |
1,8-Diiodonaphthalene Specification
The 1,8-Diiodonaphthalene, with the CAS registry number 1730-04-7, is also known as Naphthalene,1,8-diiodo-. This chemical's molecular formula is C10H6I2 and molecular weight is 379.96.
Physical properties of 1,8-Diiodonaphthalene are: (1)ACD/LogP: 5.51; (2)# of Rule of 5 Violations: 1; (3)ACD/LogD (pH 5.5): 5.51; (4)ACD/LogD (pH 7.4): 5.51; (5)ACD/BCF (pH 5.5): 9108.21; (6)ACD/BCF (pH 7.4): 9108.21; (7)ACD/KOC (pH 5.5): 23754.83; (8)ACD/KOC (pH 7.4): 23754.83; (9)#H bond acceptors: 0; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 0; (12)Polar Surface Area: 0 Å2; (13)Index of Refraction: 1.773; (14)Molar Refractivity: 69.9 cm3; (15)Molar Volume: 167.6 cm3; (16)Polarizability: 27.71×10-24 cm3; (17)Surface Tension: 57.7 dyne/cm; (18)Density: 2.265 g/cm3; (19)Flash Point: 204.3 °C; (20)Enthalpy of Vaporization: 60.81 kJ/mol; (21)Boiling Point: 384.2 °C at 760 mmHg; (22)Vapour Pressure: 9.21E-06 mmHg at 25 °C.
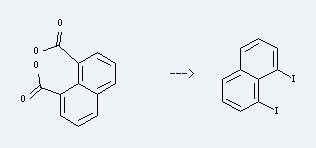
Preparation: this chemical can be prepared by Naphthalene-1,8-dicarboxylic acid with heating. This reaction will need reagent iodosobenzene diacetate, iodine and solvent CCl4 with the reaction time of 2 hours. The yield is about 80%.
Uses of 1,8-Diiodonaphthalene: it can be used to produce 1,8-(3α,4,7,7α-Tetrahydro-4,7-methanoindene-7α,8α-diyl)naphthalene at the temperature of -23 °C. It will need reagent Cyclopentadienylcopper-dimethyl sulfide and solvent Tetrahydrofuran. The yield is about 38%.
.jpg)
You can still convert the following datas into molecular structure:
(1)SMILES: Ic1cccc2cccc(I)c12
(2)InChI: InChI=1/C10H6I2/c11-8-5-1-3-7-4-2-6-9(12)10(7)8/h1-6H
(3)InChIKey: FURHMGVKKGEGMZ-UHFFFAOYAK
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 173010-79-2
- 17301-33-6
- 17301-53-0
- 17302-46-4
- 1730-25-2
- 173026-23-8
- 17302-82-8
- 173035-10-4
- 173035-11-5
- 17303-83-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View