-
Name
1-Phenyl-1-propanol
- EINECS 202-256-0
- CAS No. 93-54-9
- Article Data544
- CAS DataBase
- Density 0.994 g/cm3
- Solubility insoluble in water
- Melting Point
- Formula C9H12O
- Boiling Point 219 °C at 760 mmHg
- Molecular Weight 136.194
- Flash Point 97.3 °C
- Transport Information UN 2810
- Appearance clear colorless liquid
- Safety 23-24/25
- Risk Codes 22
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Benzylalcohol, a-ethyl- (8CI);(RS)-1-Phenylpropan-1-ol;(RS)-1-Phenylpropanol;1-Hydroxy-1-phenylpropane;1-Phenyl-1-hydroxypropane;1-Phenyl-n-propanol;1-Propanol, 1-phenyl-;Bilergon;Carbicol;Ejibil;Epatoxfen;Felicur;Felitrope;Fepar;Livonal;NSC 41708;Phenicol;Phenycholon;Phenylchol;SH 261;a-Ethylbenzenemethanol;a-Hydroxypropylbenzene;
- PSA 20.23000
- LogP 2.13000
Synthetic route

| Conditions | Yield |
|---|---|
| With methylaluminum bis(2,6-di-tert-butylphenoxide) In diethyl ether; toluene at -78℃; for 2h; | 100% |
| In diethyl ether at 0 - 20℃; | 87% |
| Stage #1: ethylmagnesium bromide; benzaldehyde In tetrahydrofuran at 30℃; for 24h; Inert atmosphere; Stage #2: With water In tetrahydrofuran Inert atmosphere; | 73% |

| Conditions | Yield |
|---|---|
| With potassium hydride; ditriptylethylborane In tetrahydrofuran for 0.0833333h; | 100% |
| With zirconium dioxide hydrate; isopropyl alcohol at 130℃; for 0.5h; Meerwein-Ponndorf-Verley Reduction; | 100% |
| With hydrogen In methanol at 40℃; for 24h; chemoselective reaction; | 100% |

| Conditions | Yield |
|---|---|
| With C35H41ClFeIrPS; hydrogen; sodium methylate In tetrahydrofuran at 25℃; under 22502.3 Torr; for 2h; Reagent/catalyst; Autoclave; Inert atmosphere; | 100% |
| With sodium tetrahydroborate; C11H18Cl2CoN2S; hydrogen In isopropyl alcohol at 100℃; under 37503.8 Torr; for 16h; Glovebox; Autoclave; | 97% |
| With 1,1'-bis(diphenylphosphino)ferrocene; [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; Butane-1,4-diol; potassium tert-butylate at 110℃; for 24h; Inert atmosphere; | 92% |
-
-
321884-15-5
2-(1-phenyl-propoxy)-tetrahydro-pyran

-
-
93-54-9
1-Phenyl-1-propanol

| Conditions | Yield |
|---|---|
| silica-supported prop-1-ylsulfonic acid In methanol | 99.6% |
| With tetra-N-butylammonium tribromide In methanol at 20℃; for 1h; | 97% |
| With tetra-N-butylammonium tribromide In methanol for 1.2h; | 97% |
| With trichloroisocyanuric acid In methanol at 20℃; for 4h; | 92% |

| Conditions | Yield |
|---|---|
| With (1R,2S)-2-aminomethyl-2-hydroxy-1,7,7-trimethylbicyclo[2.2.1]heptane In hexane for 48h; | 99% |
| With polystyrene bond triphenylphosphonium chloride In toluene at 0 - 23℃; Inert atmosphere; chemoselective reaction; | 99% |
| With 15-crown-5; sodium iodide In toluene at 0 - 23℃; for 24h; Reagent/catalyst; Solvent; Inert atmosphere; Schlenk technique; | 99% |

| Conditions | Yield |
|---|---|
| With hydrogen; TMSB; palladium In ethanol at 20℃; under 760 Torr; for 20h; | 98% |
| With riboflavin tetrabutyrate; oxygen; hydrazine hydrate In acetonitrile at 30℃; under 760.051 Torr; for 30h; | 92% |
| With oxygen; hydrazine hydrate; riboflavin 2’,3’,4’,5’-tetrabutanoate In acetonitrile at 25 - 30℃; for 30h; chemoselective reaction; | 92% |

| Conditions | Yield |
|---|---|
| With titanium tetrachloride In dichloromethane at -78 - -30℃; Product distribution; further aldehydes and tetraalkylleads; reaction with mixed tetraalkylleads; other conditions; | 98% |
| With titanium tetrachloride In dichloromethane at -78 - -30℃; | 98% |
| With titanium tetrachloride In dichloromethane at -78 - -30℃; | 96% |
-
-
64346-32-3
triethyl-butyl plumbane

-
-
100-52-7
benzaldehyde

-
A
-
93-54-9
1-Phenyl-1-propanol

-
B
-
583-03-9
1-Phenyl-1-pentanol

| Conditions | Yield |
|---|---|
| With titanium tetrachloride In dichloromethane at -78 - -30℃; | A 98% B 1.7% |

-
-
136116-42-2
1-phenyl-1-tert-butyldimethylsiloxypropane

-
-
93-54-9
1-Phenyl-1-propanol

| Conditions | Yield |
|---|---|
| With tetra-N-butylammonium tribromide In methanol for 7h; | 98% |
| With N-iodo-succinimide In methanol at 20℃; for 19h; | 95% |
| With methanol at 20℃; for 3.33333h; | 93% |
| sulfonic acid functionalized nanoporous silica In methanol at 35℃; for 12h; | 87% |
| With tetrabutyl ammonium fluoride |

| Conditions | Yield |
|---|---|
| In toluene for 0.166667h; Alkylation; reduction; Title compound not separated from byproducts.; | A 97% B 2% |


| Conditions | Yield |
|---|---|
| In toluene for 24h; Alkylation; reduction; Title compound not separated from byproducts.; | A 97% B 2% |


| Conditions | Yield |
|---|---|
| In diethyl ether Heating; | 96% |
| With diethyl ether | |
| With 2,3-Dimethylaniline; benzene |

| Conditions | Yield |
|---|---|
| With iron(III) sulfate; water In toluene at 110℃; for 1h; Ionic liquid; | 96% |
| In ethanol at 25℃; Rate constant; Kinetics; Thermodynamic data; ΔH(excit.), ΔS(excit.); |

| Conditions | Yield |
|---|---|
| With potassium hydroxide; hydrogen; Aliquat 336; palladium on activated charcoal In 2,2,4-trimethylpentane at 50℃; for 0.833333h; Product distribution; various time, solvents; also in the presence of various aromatic halides as promoters; further benzylic alcohols; | A 96% B 4% |
-
-
23355-97-7
1-phenylpropylene oxide

-
-
93-54-9
1-Phenyl-1-propanol

| Conditions | Yield |
|---|---|
| With sodium tert-pentoxide; sodium hydride; zinc(II) chloride In 1,2-dimethoxyethane at 65℃; for 6h; | 95% |
-
-
62559-30-2
1-phenylpropyl trimethylsilyl ether

-
-
93-54-9
1-Phenyl-1-propanol

| Conditions | Yield |
|---|---|
| With silica triflate In methanol for 0.0666667h; Heating; | 95% |
| With 1-butyl-3-methylimidazolium chloride at 20℃; for 1.33h; | 94% |
| With silica gel; iodic acid In water for 0.00833333h; microwave irradiation; | 90% |

-
-
93-54-9
1-Phenyl-1-propanol

| Conditions | Yield |
|---|---|
| With silica gel In methanol for 4h; Reflux; | 95% |
| With sodium hydroxide In tetrahydrofuran; diethyl ether; water at 20℃; for 0.5h; | |
| With silica gel In methanol at 60℃; for 6h; Inert atmosphere; Schlenk technique; Glovebox; | |
| With air In diethyl ether | 129 mg |
| With dihydrogen peroxide; sodium hydroxide Inert atmosphere; | 145 mg |

| Conditions | Yield |
|---|---|
| With dibutylmagnesium; 4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane In toluene at 75℃; for 24h; regioselective reaction; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: ethyl iodide With 1-butylpyridinium bromide; N-butylpyridinium tetrafluoroborate; zinc at 50℃; for 1h; Stage #2: benzaldehyde at 20℃; for 12h; | 94% |
| Stage #1: ethyl iodide With magnesium In diethyl ether Inert atmosphere; Stage #2: benzaldehyde In diethyl ether at 0 - 20℃; for 2h; Grignard Reaction; Inert atmosphere; | 84% |
| Stage #1: ethyl iodide With N-butylpyridinium tetrafluoroborate at 20℃; for 1h; Stage #2: benzaldehyde With pyridine at 20℃; for 12h; Further stages.; | 83% |
| With diethyl ether; calcium |

| Conditions | Yield |
|---|---|
| In toluene for 0.25h; Alkylation; | 94% |

| Conditions | Yield |
|---|---|
| With hydrogen In ethanol; water at 120℃; under 37503.8 Torr; chemoselective reaction; | 94% |

| Conditions | Yield |
|---|---|
| With C20H24B10Cl4FeN6; dihydrogen peroxide In methanol at 20℃; for 6h; | 93% |
| With lithium aluminium tetrahydride; 2,2'-azobis(isobutyronitrile); oxygen Kinetics; relative chain propagation rates; | |
| With NADPH In dimethyl sulfoxide at 30℃; pH=7.4; Product distribution; Further Variations:; Reagents; | 99 % Chromat. |

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)nickel (0); tri-tert-butyl phosphine; 1-(Prop-2-enyl)-1,2,3,4,5-pentamethylcyclopenta-2,4-dien In hexane; toluene at 0℃; for 24h; | 93% |
| With tri-tert-butyl phosphine; bis(1,5-cyclooctadiene)nickel (0) In hexane; water at 20℃; for 20h; Product distribution; Further Variations:; Catalysts; Reagents; Solvents; | 81% |
| With tetra-(n-butyl)ammonium iodide In tetrahydrofuran; N,N-dimethyl-formamide for 7h; Ambient temperature; electrolysis with Pt/Cu electrodes; | 74% |

| Conditions | Yield |
|---|---|
| With C35H41FePRhS; hydrogen; sodium methylate In tetrahydrofuran at 82℃; under 22502.3 Torr; for 16h; Autoclave; Inert atmosphere; | A 7% B 93% |
| With triethylamine; palladium on activated charcoal In toluene at 150℃; for 3h; | A 5 % Spectr. B 91 % Spectr. |
| With dichloro(η3:η2:η3-dodeca-2,6,10-triene-1,12-diyl)ruthenium(IV); water; sodium formate at 100℃; for 24h; Inert atmosphere; | A 82 %Chromat. B n/a |

| Conditions | Yield |
|---|---|
| With alkaline hydrogen peroxide; lithium triethylborohydride In tetrahydrofuran for 1h; | 92% |
-
-
1131-80-2, 1201-93-0, 31919-75-2
(E)-3-(N,N-dimethylamino)-1-phenyl-2-propen-1-one

-
-
93-54-9
1-Phenyl-1-propanol

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In ethanol at 20℃; for 12h; Reagent/catalyst; Temperature; | 92% |
-
-
1190991-23-1
potassium trifluoro(1-phenylpropan-2-yl)borate

-
-
93-54-9
1-Phenyl-1-propanol

| Conditions | Yield |
|---|---|
| With rose bengal; triethylamine In ethanol at 25℃; for 12h; Schlenk technique; Irradiation; | 91% |

| Conditions | Yield |
|---|---|
| With lithium In tetrahydrofuran at -30℃; for 0.25h; | 90% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 0.0833333h; | 90% |
| In tetrahydrofuran at 20℃; further carbonyl compounds, further solvents, further Grignard/TDA-1 complexes; | 90% |

| Conditions | Yield |
|---|---|
| With silica-supported Jones reagent In dichloromethane for 0.00269444h; | 100% |
| With ruthenium trichloride; iodobenzene; potassium peroxomonosulfate In water; acetonitrile at 20℃; for 0.3h; | 100% |
| With ruthenium trichloride; iodobenzene; potassium peroxymonosulfate In water; acetonitrile at 20℃; for 0.3h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| indium(III) chloride at 20℃; for 1h; | 100% |
| With pyridine | 99% |
| With SiO2-supported Co(II) Salen complex catalyst at 50℃; for 0.75h; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: ethyl iodide With magnesium; N-butylpyridinium tetrafluoroborate at 20℃; for 1h; Stage #2: 1-Phenyl-1-propanol With magnesium iodide at 20℃; for 12h; Further stages.; | 100% |

| Conditions | Yield |
|---|---|
| In diethyl ether at 0 - 20℃; | 100% |
-
-
93-54-9
1-Phenyl-1-propanol

-
-
144-79-6
chloromethyldiphenylsilane

-
-
60632-85-1
1-phenylpropan-1-yl diphenylmethylsilyl ether

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 20℃; for 2h; Inert atmosphere; | 100% |
-
-
110-87-2
3,4-dihydro-2H-pyran

-
-
93-54-9
1-Phenyl-1-propanol

-
-
321884-15-5
2-(1-phenyl-propoxy)-tetrahydro-pyran

| Conditions | Yield |
|---|---|
| silica-supported prop-1-ylsulfonic acid In acetonitrile for 0.166667h; | 99.8% |
| With C8H15N2O3S(1+)*Cl5Zn2(1-) In neat (no solvent) at 20℃; for 0.75h; Green chemistry; | 92% |
| With trichloroisocyanuric acid at 60 - 80℃; for 6h; | 90% |
| bismuth(III) nitrate In dichloromethane at 20℃; for 0.3h; | 90% |
| With tetra-N-butylammonium tribromide In dichloromethane at 20℃; for 0.41h; | 85% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-Phenyl-1-propanol With sodium hydride In tetrahydrofuran at 20℃; for 1h; Stage #2: methyl iodide at 20℃; | 99.2% |

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane; acetonitrile; sodium iodide In hexane for 24h; Ambient temperature; | 99% |
| With chloro-trimethyl-silane; acetonitrile; sodium iodide In hexane for 24h; Ambient temperature; | 99% |
| With hydrogen In ethanol at 80℃; under 2250.23 Torr; for 3h; Catalytic behavior; Temperature; Solvent; Inert atmosphere; | 94% |
-
-
108-22-5
Isopropenyl acetate

-
-
93-54-9
1-Phenyl-1-propanol

-
-
2114-29-6, 83808-03-1, 83860-48-4, 84275-44-5
(R)-1-phenylpropyl acetate

| Conditions | Yield |
|---|---|
| With dicarbonylchlorido(pentabenzylcyclopentadienyl)ruthenium; potassium tert-butylate; sodium carbonate In tetrahydrofuran; toluene at 23℃; for 9h; Inert atmosphere; dynamic kinetic resolution; Enzymatic reaction; optical yield given as %ee; enantioselective reaction; | 99% |
| With Candida antarctica lipase B; potassium tert-butylate; sodium carbonate; [chlorodicarbonyl(η-pentaphenylcyclopentadienyl)]Ru(II) In toluene at 20℃; for 17h; | 90% |
| Stage #1: Isopropenyl acetate; 1-Phenyl-1-propanol With Novozym 435 In toluene at 60℃; for 1h; Enzymatic reaction; Stage #2: In toluene at 60℃; for 21h; | n/a |
-
-
93-54-9
1-Phenyl-1-propanol

-
-
10316-10-6
(-)-(S)-(1-chloropropyl)benzene

| Conditions | Yield |
|---|---|
| With 1,3,5-trichloro-2,4,6-triazine; dimethyl sulfoxide at 20℃; for 0.416667h; chemoselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-Phenyl-1-propanol With allylchloro[1,3-bis(2,6-di-isopropylphenyl)imidazol-2-ylidine]palladium(II); para-chlorotoluene; potassium tert-butylate In toluene at 40℃; for 2.5h; Inert atmosphere; Stage #2: 2,6-dimethyl-1-chlorobenzene In toluene at 80℃; for 2h; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With allylchloro[1,3-bis(2,6-di-isopropylphenyl)imidazol-2-ylidine]palladium(II); potassium tert-butylate In toluene at 80℃; for 1.5h; Inert atmosphere; | 99% |
-
-
93-54-9
1-Phenyl-1-propanol

-
-
5123-13-7
5,5-dimethyl-2-phenyl-1,3,2-dioxaborinane

-
-
5180-33-6
1,1-Diphenyl-propan-1-ol

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)nickel (0); potassium tert-butylate; chlorobenzene; cesium fluoride; 1,3-bis[2,6-diisopropylphenyl]imidazolium chloride In toluene at 80℃; for 4h; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-Phenyl-1-propanol; benzoic acid With N-Bromosuccinimide In 1,4-dioxane at 60℃; for 1h; Sealed tube; Stage #2: With 1,8-diazabicyclo[5.4.0]undec-7-ene In 1,4-dioxane at 60℃; for 2h; Reagent/catalyst; Solvent; Temperature; | 99% |
| Stage #1: 1-Phenyl-1-propanol; benzoic acid With N-Bromosuccinimide In 1,4-dioxane at 60℃; for 1h; Stage #2: With 1,8-diazabicyclo[5.4.0]undec-7-ene In 1,4-dioxane for 2h; | 99% |
| Stage #1: 1-Phenyl-1-propanol; benzoic acid With N-Bromosuccinimide In 1,4-dioxane at 60℃; for 1h; Stage #2: With 1,8-diazabicyclo[5.4.0]undec-7-ene In 1,4-dioxane for 2h; | 99% |
| With tert.-butylhydroperoxide; tetra-(n-butyl)ammonium iodide In water; ethyl acetate at 100℃; for 12h; Catalytic behavior; Reagent/catalyst; Solvent; Schlenk technique; | 72% |

| Conditions | Yield |
|---|---|
| With C24H24IrN4O4(1+)*BF4(1-); sodium t-butanolate at 140℃; for 24h; Sealed tube; Inert atmosphere; | 99% |
| With caesium carbonate at 150℃; for 24h; | 87% |
| With carbonylhydrido(tetrahydroborato)[bis(2-diphenylphosphinoethyl)amino]ruthenium(II); sodium methylate at 150℃; for 20h; Inert atmosphere; Autoclave; Glovebox; Green chemistry; | 83 %Spectr. |
| With trimethylamine-N-oxide; (1,4-dimethyl-5,7-diphenyl-1,2,3,4-tetrahydro-6H-cyclopenta[b]pyrazin-6-one) irontricarbonyl complex3; sodium hydroxide at 130℃; for 24h; | 12 %Spectr. |

| Conditions | Yield |
|---|---|
| With potassium hydroxide | 98.4% |
| With dmap; triethylamine In tetrahydrofuran; dichloromethane Heating; | 84% |
-
-
93-54-9
1-Phenyl-1-propanol

-
-
999-97-3
1,1,1,3,3,3-hexamethyl-disilazane

-
-
62559-30-2
1-phenylpropyl trimethylsilyl ether

| Conditions | Yield |
|---|---|
| With polyvinylpolypyrrolidonium tribromide In acetonitrile at 20℃; for 0.116667h; | 98% |
| With L-Aspartic acid In acetonitrile at 20℃; for 0.0666667h; | 98% |
| With bismuth(lll) trifluoromethanesulfonate at 20℃; for 0.0333333h; Neat (no solvent); | 96% |

| Conditions | Yield |
|---|---|
| With aluminum dodecatungstophosphate at 20℃; for 0.166667h; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: succinic acid anhydride; 1-Phenyl-1-propanol With dmap In dichloromethane at 20℃; for 2h; Inert atmosphere; Stage #2: With hydrogenchloride In water | 98% |
| With dmap In dichloromethane at 20℃; Inert atmosphere; |
-
-
95-49-8
2-methylchlorobenzene

-
-
93-54-9
1-Phenyl-1-propanol

-
-
53423-27-1
2-(2-methylphenyl)-1-phenylpropan-1-one

| Conditions | Yield |
|---|---|
| With allylchloro[1,3-bis(2,6-di-isopropylphenyl)imidazol-2-ylidine]palladium(II); potassium tert-butylate In toluene at 80℃; for 4h; Inert atmosphere; | 98% |
1-Phenyl-1-propanol Chemical Properties
Molar mass:136.19098g/mol
Structure of 1-Phenyl-1-propanol(93-54-9):
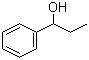
Synonyms of 1-Phenyl-1-propanol(93-54-9):.alpha.-Hydroxypropylbenzene;alpha-ethylbenzyl alcohol;1-Phenyl-1-propanol;Gallenperlen;Phenycholon;1-Phenyl-1-Hydroxypropane;1-Phenylpropan-1-ol;1-phenyl-propan-1-ol;1-Propanol, 1-phenyl-
Density:0.994 g/cm3
Flash Point:97.3 °C
Boiling Point:219 °C at 760 mmHg
Index of Refraction:1.524
Vapour Pressure:0.0707 mmHg at 25°C
Water solubility:insoluble
Appearance:Colorless oily liquid
1-Phenyl-1-propanol Uses
1-Phenyl-1-propanol Production
1-Phenyl-1-propanol Toxicity Data With Reference
| 1. | orl-rat LD50:1600 mg/kg | ARZNAD Arzneimittel-Forschung. Drug Research. 12 (1962),347. | ||
| 2. | orl-mus LD50:500 mg/kg | AIPTAK Archives Internationales de Pharmacodynamie et de Therapie. 116 (1958),154. | ||
| 3. | scu-mus LD50:700 mg/kg | AIPTAK Archives Internationales de Pharmacodynamie et de Therapie. 116 (1958),154. |
Other: See actual entry in RTECS for complete information.The toxicological properties have not been fully investigated. Mutagenicity: Ames-test: negative.
1-Phenyl-1-propanol Safety Profile
Hazard Codes:Xn

Risk Statements:
22: Harmful if swallowed
Safety Statements:
23: Do not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer)
24: Avoid contact with skin
25: Avoid contact with eyes
1-Phenyl-1-propanol Specification
Conditions to Avoid:Incompatible materials.
Chemical Stability:Stable under normal temperatures and pressures.
Incompatibilities with Other Materials:Incompatible materials, acid anhydrides, acid chlorides, acids.
Hazardous Decomposition Products: carbon dioxide,Carbon monoxide.
Related Products
- 1-Phenyl-1-propanol
- 93549-15-6
- 93-55-0
- 935521-01-0
- 935525-13-6
- 935534-47-7
- 93553-81-2
- 93554-80-4
- 935-56-8
- 93-56-1
- 935661-15-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View