-
Name
1-Pyrenebutanol
- EINECS
- CAS No. 67000-89-9
- Article Data14
- CAS DataBase
- Density 1.217 g/cm3
- Solubility
- Melting Point 80-83 °C(lit.)
- Formula C20H18O
- Boiling Point 488.4 °C at 760 mmHg
- Molecular Weight 274.362
- Flash Point 205.4 °C
- Transport Information
- Appearance
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 4-(1-Pyrenyl)butanol;
- PSA 20.23000
- LogP 4.89900
Synthetic route
-
-
70570-29-5
4-(1-pyrene)butyric acid methyl ester

-
-
67000-89-9
4-pyrenylbutanol

| Conditions | Yield |
|---|---|
| With diisobutylaluminium hydride In tetrahydrofuran; hexane at 0 - 20℃; Inert atmosphere; | 99% |
| With lithium aluminium tetrahydride In tetrahydrofuran at 0 - 20℃; |
-
-
3443-45-6
1-pyrenebutyric acid

-
-
67000-89-9
4-pyrenylbutanol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride | 95% |
| With dimethylsulfide borane complex In tetrahydrofuran at 0 - 20℃; | 90.2% |
| With borane-THF In tetrahydrofuran at 20℃; Cooling with ice; | 79% |
-
-
59275-39-7
4-(1-pyrene)butanoic acid ethyl ester

-
-
67000-89-9
4-pyrenylbutanol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran at 20℃; for 4h; | 88% |

-
-
67000-89-9
4-pyrenylbutanol

| Conditions | Yield |
|---|---|
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone 1.) methanol, -78 deg C, 2.) abs. toluene, reflux, 1h.; Yield given. Multistep reaction; |

-
-
67000-89-9
4-pyrenylbutanol

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide | 100 % Chromat. |
-
-
129-00-0
pyrene

-
-
67000-89-9
4-pyrenylbutanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1.) lithium wire / 1.)-78 deg C, 12 h., 2.) room temp., 12 h. 2: 2.) DDQ / 1.) methanol, -78 deg C, 2.) abs. toluene, reflux, 1h. View Scheme | |
| Multi-step reaction with 4 steps 1.1: aluminum (III) chloride / nitrobenzene / 18 h / 0 - 20 °C 2.1: potassium hydroxide; hydrazine / diethylene glycol / 2 h / Heating / reflux 2.2: 0 °C 3.1: thionyl chloride / 0 - 20 °C 4.1: lithium aluminium tetrahydride / tetrahydrofuran / 0 - 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| In acetonitrile for 0.166667h; pH=4.8; |

-
-
7499-60-7
γ-oxo-1-pyrenebutyric acid

-
-
67000-89-9
4-pyrenylbutanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: potassium hydroxide; hydrazine / diethylene glycol / 2 h / Heating / reflux 1.2: 0 °C 2.1: thionyl chloride / 0 - 20 °C 3.1: lithium aluminium tetrahydride / tetrahydrofuran / 0 - 20 °C View Scheme |
-
-
10436-25-6
11-(tert-butoxycarbonylamino)undecanoic acid

-
-
67000-89-9
4-pyrenylbutanol

-
-
1416547-75-5
C36H47NO4

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 120h; | 99% |
-
-
67000-89-9
4-pyrenylbutanol

-
-
124-63-0
methanesulfonyl chloride

-
-
205488-04-6
4-(pyren-1-yl)butyl methanesulfonate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane Cooling with ice; | 98% |
| With triethylamine In dichloromethane at 0℃; for 12h; | 96% |
| With triethylamine In dichloromethane Cooling with ice; | 91.2% |
| With triethylamine In dichloromethane at 20℃; for 2h; Inert atmosphere; | 91% |
| With triethylamine In dichloromethane at 20℃; for 4h; |
-
-
67000-89-9
4-pyrenylbutanol

| Conditions | Yield |
|---|---|
| With pyridinium chlorochromate In dichloromethane at 20℃; for 2h; | 97% |
| With dipyridinium dichromate In dichloromethane at 20℃; Inert atmosphere; | 97% |
| With pyridinium chlorochromate In dichloromethane at 20℃; | 95% |
-
-
67000-89-9
4-pyrenylbutanol

-
-
117846-05-6
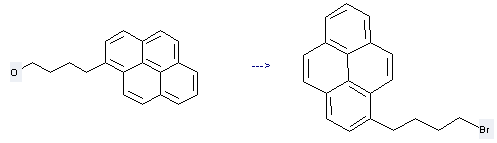
1-bromo-4-(1-pyrenyl)butane

| Conditions | Yield |
|---|---|
| With carbon tetrabromide; triphenylphosphine In acetonitrile at 25℃; for 0.333333h; Appel Halogenation; | 96% |
| With carbon tetrabromide; triphenylphosphine In dichloromethane Bromination; substitution; | 90% |
| With carbon tetrabromide; triphenylphosphine In tetrahydrofuran | 90% |
-
-
67000-89-9
4-pyrenylbutanol

-
-
201611-92-9
4-cyano-4-(thiobenzoylthio)pentanoic acid

-
-
1048735-14-3
4-cyano-4-methyl-4-thiobenzoylsulfanyl-butyric acid 4-pyren-1-yl-butyl ester

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In tetrahydrofuran; dichloromethane at 0 - 20℃; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-pyrenylbutanol In dichloromethane Alkaline conditions; Cooling with ice; Stage #2: 2-Bromoacetyl bromide In dichloromethane at 20℃; for 24h; Cooling with ice; | 92% |
-
-
71310-21-9
11-mercaptounadecanoic acid

-
-
67000-89-9
4-pyrenylbutanol

| Conditions | Yield |
|---|---|
| With hafnium/THF In toluene for 48h; Reflux; Molecular sieve; | 89% |
-
-
42333-78-8
2,4,6-tri(4-pyridyl)-1,3,5-triazine

-
-
1039768-31-4
[Ru2(η6-p-cymene)2(C6H2O4)Cl2]

-
-
67000-89-9
4-pyrenylbutanol

-
-
2923-28-6
silver trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| In methanol byproducts: AgCl; Ru complex and AgCF3SO3 stirred in MeOH for 2 h; filtered into suspn. ofN3C3(C5H4N)3 and pyrenyl in MeOH; stirred at room temp. for 18 h; solvent removed under reduced pressure; dissolved in CH2Cl2; filtered; vol. of filtrate reduced; pptd. with Et2O; collected by filtration; | 88% |


| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane | 88% |
-
-
67000-89-9
4-pyrenylbutanol

-
-
1448599-87-8
3-oxo-3-(pent-4-yn-1-yloxy)propanoic acid

-
-
1448599-89-0
pent-4-yn-1-yl (4-(pyren-1-yl)butyl) malonate

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0℃; for 2h; Cooling with ice; Inert atmosphere; | 87% |
-
-
67000-89-9
4-pyrenylbutanol

-
-
94715-74-9
O-(2-Acetamido-3,4,6-tri-O-acetyl-2-desoxy-α-D-glucopyranosyl)trichloracetimidat

-
-
1160291-41-7
4-(1-pyrenyl)butyl 2-acetamido-3,4,6-tri-O-acetyl-2-deoxy-β-D-glucopyranoside

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate In dichloromethane at 20℃; for 3h; | 85% |
-
-
67000-89-9
4-pyrenylbutanol

-
-
227000-85-3
N-tert-butyl-O-[1-(4-chloromethyl-phenyl)-ethyl]-N-(2-methyl-1-phenyl-propyl)-hydroxylamine

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran for 16h; Heating; | 84% |

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran at 20℃; for 24h; | 84% |
-
-
1119-62-6
3,3'-dithiobis(propionic acid)

-
-
67000-89-9
4-pyrenylbutanol

-
-
1259437-62-1
3-((3-oxo-3-(4-(pyren-1-yl)butoxy)propyl)disulfanyl)propanoic acid

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In tetrahydrofuran at 20℃; for 20h; | 81.3% |
-
-
67000-89-9
4-pyrenylbutanol

-
-
106-96-7
propargyl bromide

-
-
1211548-30-9
1-(4-(prop-2-yn-1-yloxy)butyl)pyrene

| Conditions | Yield |
|---|---|
| Stage #1: 4-pyrenylbutanol With sodium hydride In tetrahydrofuran at 0℃; for 0.5h; Inert atmosphere; Stage #2: propargyl bromide In tetrahydrofuran; toluene at 0℃; Inert atmosphere; Reflux; Darkness; | 81% |
| Stage #1: 4-pyrenylbutanol With sodium hydride In tetrahydrofuran at 0℃; for 1h; Inert atmosphere; Stage #2: propargyl bromide In tetrahydrofuran; toluene at 0℃; for 1h; Inert atmosphere; Darkness; | 69% |
-
-
67000-89-9
4-pyrenylbutanol

-
-
920-46-7
Methacryloyl chloride

-
-
71254-27-8
4-(pyren-1-yl)butyl methacrylate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 1h; Inert atmosphere; | 80% |
| With triethylamine In dichloromethane at 20℃; | |
| With triethylamine In dichloromethane at 0℃; for 2h; |
-
-
7719-09-7
thionyl chloride

-
-
67000-89-9
4-pyrenylbutanol

-
-
864767-17-9
[(C5H5)TiCl2(C5H4C(CH3)2CH2C(O)O(CH2)4C16H9)]

| Conditions | Yield |
|---|---|
| With NaH In dichloromethane Ti complex was reacted with SOCl2 at room temp. for 1 h; heated at 50°C for 2 h in vac.; dissolved in CH2Cl2; transferred to mixt. of NaHand alcohol in CH2Cl2; stirred at room temp. for 16 h; filtered through Celite; crystd. (CH2Cl2/pentane); | 79% |

-
-
67000-89-9
4-pyrenylbutanol

-
-
4101-68-2
1,10-dibromodecane

-
-
244013-58-9
1-[4-(10-bromo-decyloxy)-butyl]-pyrene

| Conditions | Yield |
|---|---|
| Stage #1: 4-pyrenylbutanol With potassium tert-butylate In toluene at 100℃; for 1h; Stage #2: 1,10-dibromodecane In toluene for 16h; | 79% |
-
-
88-13-1
3-Thiophene carboxylic acid

-
-
67000-89-9
4-pyrenylbutanol

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; for 12.5h; Inert atmosphere; | 79% |
-
-
6964-21-2
thiophen-3-yl-acetic acid

-
-
67000-89-9
4-pyrenylbutanol

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; for 12.5h; Inert atmosphere; | 76% |
-
-
67000-89-9
4-pyrenylbutanol

-
-
107-96-0
3-mercaptopropionic acid

-
-
1041403-51-3
4-(pyren-1-yl)butyl 3-mercaptopropionate

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In benzene Inert atmosphere; Reflux; | 75% |
-
-
6089-09-4
4-pentynoic acid

-
-
67000-89-9
4-pyrenylbutanol

-
-
1213789-09-3
4-(pyren-1-yl)butyl pent-4-ynoate

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane | 72% |
-
-
67000-89-9
4-pyrenylbutanol

-
-
13734-34-4
N-tert-butoxycarbonyl-L-phenylalanine

-
-
1400924-49-3
Boc-Phe-Pyrene

| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine; ethyl cyanoglyoxylate-2-oxime In N,N-dimethyl-formamide at 0℃; for 2h; | 71% |
-
-
67000-89-9
4-pyrenylbutanol

-
-
600727-96-6
2,6-bis(pyrazole-1-yl)pyridine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; for 72h; Inert atmosphere; Schlenk technique; | 69% |
-
-
7703-74-4
2,6-bis-(bromomethyl)pyridine

-
-
67000-89-9
4-pyrenylbutanol

| Conditions | Yield |
|---|---|
| Stage #1: 4-pyrenylbutanol With sodium hydride In tetrahydrofuran at 20℃; for 0.5h; Inert atmosphere; Cooling with ice; Stage #2: 2,6-bis-(bromomethyl)pyridine In tetrahydrofuran at 20℃; for 4h; Inert atmosphere; | 68% |
| With sodium hydride In tetrahydrofuran; mineral oil for 18h; Reflux; Inert atmosphere; | 62% |

| Conditions | Yield |
|---|---|
| With potassium hydride In dichloromethane at 0 - 20℃; for 6h; | 62% |

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 4h; | 60% |
1-Pyrenebutanol Specification
The 1-Pyrenebutanol, with the CAS registry number 67000-89-9, is also known as 4-(Pyren-1-yl)butan-1-ol. It belongs to the product categories of Alcohols; C9 to C30; Oxygen Compounds. This chemical's molecular formula is C20H18O and molecular weight is 274.36. Its systematic name is called 4-pyren-1-ylbutan-1-ol. The product should be sealed and stored in cool and dry place.
Physical properties of 1-Pyrenebutanol: (1)ACD/LogP: 5.36; (2)# of Rule of 5 Violations: 1; (3)ACD/LogD (pH 5.5): 5.36; (4)ACD/LogD (pH 7.4): 5.36; (5)ACD/BCF (pH 5.5): 6941.32; (6)ACD/BCF (pH 7.4): 6941.32; (7)ACD/KOC (pH 5.5): 19556.7; (8)ACD/KOC (pH 7.4): 19556.7; (9)#H bond acceptors: 1; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 5; (12)Index of Refraction: 1.761; (13)Molar Refractivity: 92.81 cm3; (14)Molar Volume: 225.3 cm3; (15)Surface Tension: 58.2 dyne/cm; (16)Density: 1.217 g/cm3; (17)Flash Point: 205.4 °C; (18)Enthalpy of Vaporization: 79.46 kJ/mol; (19)Boiling Point: 488.4 °C at 760 mmHg; (20)Vapour Pressure: 2.37E-10 mmHg at 25°C.
Preparation of 1-Pyrenebutanol: this chemical can be prepared by 4-(1-pyrenyl)butanol. This reaction is a kind of Bromination//substitution. It will need reagents tetrabromomethane, triphenylphosphine and solvent CH2Cl2. The yield is about 90%.
When you are using this chemical, please be cautious about it as the following:
This chemical may cause inflammation to the skin or other mucous membranes. It is irritating to eyes, respiratory system and skin. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. Whenever you will contact it, please wear suitable protective clothing.
You can still convert the following datas into molecular structure:
(1)SMILES: OCCCCc4ccc2ccc1cccc3c1c2c4cc3
(2)InChI: InChI=1/C20H18O/c21-13-2-1-4-14-7-8-17-10-9-15-5-3-6-16-11-12-18(14)20(17)19(15)16/h3,5-12,21H,1-2,4,13H2
(3)InChIKey: MRENSFROWALQNU-UHFFFAOYAP
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View