-
Name
17-Methyltestosterone
- EINECS 200-366-3
- CAS No. 58-18-4
- Article Data57
- CAS DataBase
- Density 1.1 g/cm3
- Solubility water: ≤0.5 mg/mL
- Melting Point 162-168 °C(lit.)
- Formula C20H30O2
- Boiling Point 434.4 °C at 760 mmHg
- Molecular Weight 302.457
- Flash Point 185.3 °C
- Transport Information
- Appearance white to slightly yellowish-white crystalline
- Safety 53-36/37-45-24/25-22
- Risk Codes 45-63-22-20-19-11-61-60-36/37/38
-
Molecular Structure
-
Hazard Symbols
 T,
T, Xn,
Xn, Xi,
Xi, F
F
- Synonyms Androst-4-en-3-one,17b-hydroxy-17-methyl- (8CI);17a-Methyl-17b-hydroxyandrost-4-en-3-one;17a-Methyl-3-oxo-4-androsten-17b-ol;17a-Methyltestosterone;17b-Hydroxy-17a-methylandrost-4-ene-3-one;Android;Androsan;Androst-4-ene-17a-methyl-17b-ol-3-one;CDB 110;Glosso-Sterandryl;Homandren;Mesterone;Metandren;Oreton Methyl;Perandren;Steronyl;Synandrotabs;Testora;Testoviron (tablet);Testred;
- PSA 37.30000
- LogP 4.26930
Synthetic route
-
-
111222-36-7
(8R,9S,10R,13S,14S,17S)-10,13,17-Trimethyl-17-(tetrahydro-furan-2-yloxy)-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-cyclopenta[a]phenanthren-3-one

-
-
58-18-4
17-methyltestosterone

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In ethanol for 1h; Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| With <(C4Ph4COHOCC4Ph4)(μ-H)><(CO)4Ru2>; acetone at 56℃; for 18h; | 93% |
| With chromium(VI) oxide; bromine; acetic acid Behandeln des Reaktionsprodukts mit CrCl2 in wss.Methanol unter Kohlendioxyd; | |
| With aluminum tri-tert-butoxide; acetone; benzene |

-
-
58-18-4
17-methyltestosterone

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water; toluene at 60 - 65℃; Solvent; Reagent/catalyst; | 81.6% |

-
A
-
58-18-4
17-methyltestosterone

-
B
-
18167-94-7, 35937-38-3, 132830-76-3, 65-04-3
5α-17β-hydroxy-17α-methyl-Δ1-androsten-3-one

| Conditions | Yield |
|---|---|
| With 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione In dimethyl sulfoxide at 65℃; for 24h; | A 10% B 74% |
| With 1-n-butyl-3-methylimidazolim bromide; 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione at 65℃; for 22h; | A 10% B 74% |

| Conditions | Yield |
|---|---|
| With thallium(III) nitrate trihydrate In diethylene glycol dimethyl ether for 24h; Ambient temperature; | 34% |
-
-
110-86-1
pyridine

-
-
521-10-8
methylandrostenediol

-
-
76-83-5
trityl chloride

-
-
58-18-4
17-methyltestosterone

| Conditions | Yield |
|---|---|
| Erhitzen des Reaktionsprodukts mit Kupfer-Spaenen unter vermindertem Druck auf 250-300grad; |

-
-
972-46-3
3-ethoxyandrosta-3,5-dien-17-one

-
-
75-16-1
methylmagnesium bromide

-
-
58-18-4
17-methyltestosterone

| Conditions | Yield |
|---|---|
| With diethyl ether; toluene anschliessend Behandeln mit wss.NH4Cl und Behandeln des Reaktionsprodukts mit wss.Saeure; |

| Conditions | Yield |
|---|---|
| With aluminum isopropoxide; toluene |
-
-
521-10-8
methylandrostenediol

-
-
108-94-1
cyclohexanone

-
-
555-31-7
aluminum isopropoxide

-
-
108-88-3
toluene

-
-
58-18-4
17-methyltestosterone


| Conditions | Yield |
|---|---|
| With pyridine Erhitzen des Reaktionsprodukts mit Kupfer-Spaenen unter vermindertem Druck auf 250-300grad; | |
| With pyridine Erhitzen des Reaktionsprodukts mit Kupfer-Spaenen unter vermindertem Druck auf 250-300grad; |
-
-
521-10-8
methylandrostenediol

-
-
555-31-7
aluminum isopropoxide

-
-
67-64-1
acetone

-
-
71-43-2
benzene

-
-
58-18-4
17-methyltestosterone

-
-
521-10-8
methylandrostenediol

-
-
556-91-2
aluminum tri-tert-butoxide

-
-
67-64-1
acetone

-
-
71-43-2
benzene

-
-
58-18-4
17-methyltestosterone


| Conditions | Yield |
|---|---|
| With diethyl ether; toluene anschliessend mit wss.NH4Cl und Behandeln des Reaktionsprodukts mit wss.Saeure; |

| Conditions | Yield |
|---|---|
| Behandeln des Reaktionsprodukts mit wss.-methanol. CrCl2-Loesung unter Kohlendioxyd; | |
| Behandeln des Reaktionsprodukts in Aethanol und Benzol oder in Eisessig mit Zink-Pulver; | |
| Behandeln des Reaktionsprodukts in Aethanol und Benzol oder in Eisessig mit Zink-Pulver; |




| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: diethyl ether 2: bromine; acetic acid; CrO3 / Behandeln von Loesungen des Reaktionsprodukts in Aethanol und Benzol oder in Essigsaeure mit Zink-Pulver View Scheme | |
| Multi-step reaction with 2 steps 1: diethyl ether 2: bromine; acetic acid; CrO3 / Behandeln des Reaktionsprodukts mit CrCl2 in wss.Methanol unter Kohlendioxyd View Scheme |
-
-
57-83-0
Progesterone

-
A
-
1096-38-4
16-dehydroprogesterone

-
B
-
58-18-4
17-methyltestosterone

-
C
-
145-13-1
Pregnenolone

-
D
-
64-85-7
21-Hydroxyprogesterone

-
E
-
17398-60-6
pregna-4,7-diene-3,20-dione

-
F
-
30802-24-5
pregnane-3,6,20-trione

-
G
-
7350-00-7
pregnane-3,20-dione

| Conditions | Yield |
|---|---|
| With Hortaea werneckii B-763 at all growth phases In YNB growth medium; N,N-dimethyl-formamide at 28℃; for 24h; Enzymatic reaction; |


| Conditions | Yield |
|---|---|
| Stage #1: Chlorodifluoromethane With iodine; triethylamine In tetrahydrofuran at 40 - 45℃; for 1h; Stage #2: C22H36O3 In tetrahydrofuran; toluene at 1 - 45℃; for 24h; | 33.9 g |

-
-
58-18-4
17-methyltestosterone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: pyridine; trichlorophosphate / N,N-dimethyl-formamide 2: sodium hydroxide; sodium tetrahydroborate 3: manganese(IV) oxide View Scheme |

| Conditions | Yield |
|---|---|
| With manganese(IV) oxide |
-
-
38521-84-5
3-acetyloxypregna-5,16-diene-20-one

-
-
58-18-4
17-methyltestosterone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: hydroxylamine hydrochloride 2: pyridine; trichlorophosphate / N,N-dimethyl-formamide 3: sodium hydroxide; sodium tetrahydroborate 4: manganese(IV) oxide View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: hydrogenchloride / ethanol / 20 - 25 °C 2.1: magnesium / tetrahydrofuran / 45 - 50 °C 2.2: 50 - 55 °C 3.1: hydrogenchloride / toluene; water / 60 - 65 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: hydrogenchloride / ethanol / 20 - 25 °C 2.1: magnesium / diethyl ether / 35 - 40 °C 2.2: 50 - 55 °C 3.1: hydrogenchloride / toluene; water / 60 - 65 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: magnesium / tetrahydrofuran / 45 - 50 °C 1.2: 50 - 55 °C 2.1: hydrogenchloride / toluene; water / 60 - 65 °C View Scheme |
-
-
109-99-9
tetrahydrofuran

-
-
58-18-4
17-methyltestosterone

-
-
111222-36-7
(8R,9S,10R,13S,14S,17S)-10,13,17-Trimethyl-17-(tetrahydro-furan-2-yloxy)-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-cyclopenta[a]phenanthren-3-one

| Conditions | Yield |
|---|---|
| With tetrachloromethane; manganese at 65℃; for 8h; | 91% |
| With ceric triethylammonium nitrate In toluene at 100℃; for 8h; | 40% |

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether; potassium tert-butylate; oxygen In toluene at -25℃; for 4h; | 91% |
-
-
58-18-4
17-methyltestosterone

-
-
2931-65-9
17β-hydroxy-17α-methyl-3,5-seco-4-nor-5-oxo-androstan-3-oic acid

| Conditions | Yield |
|---|---|
| With ozone at -31 - -29℃; for 1.5h; Inert atmosphere; | 87% |
| With acetic acid Ozonolyse; | |
| With ruthenium(IV) oxide; sodium periodate In tetrachloromethane; water; acetone for 0.5h; | 10.96 g |
-
-
58-18-4
17-methyltestosterone

-
A
-
138513-20-9
(1R,8S,9S,10R,13S,14S,17S)-1,17-Dihydroxy-10,13,17-trimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-1H-2-oxa-cyclopenta[a]phenanthren-3-one

-
B
-
110716-65-9
(1S,8S,9S,10R,13S,14S,17S)-1,17-Dihydroxy-10,13,17-trimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-1H-2-oxa-cyclopenta[a]phenanthren-3-one

| Conditions | Yield |
|---|---|
| With crown ether; potassium tert-butylate; oxygen In toluene 1.) -25 deg C, 1.5-4 h, 2.) room temperature, 3 d; Title compound not separated from byproducts; | A n/a B 85% |
-
-
58-18-4
17-methyltestosterone

-
-
107-21-1
ethylene glycol

-
-
1099-72-5
3,3-(ethylenedioxy)-17α-methyl-5-androsten-17β-ol

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In benzene for 4.5h; Heating; | 82% |
| With toluene-4-sulfonic acid; benzene | |
| With boron trifluoride diethyl etherate; orthoformic acid triethyl ester In chloroform at 30 - 35℃; for 9h; Solvent; | 22.3 g |

| Conditions | Yield |
|---|---|
| With tert-butyldimethylsilyl chloride; 2,3-dicyano-5,6-dichloro-p-benzoquinone In 1,4-dioxane at 0 - 20℃; for 48h; | 78% |
| With selenium(IV) oxide | |
| mit Hilfe von Didymella lycopersici; |
-
-
58-18-4
17-methyltestosterone

-
-
96992-13-1
6-Ketomethyltestosterone

| Conditions | Yield |
|---|---|
| With potassium hydroxide; tetrazolium blue In ethanol Heating; | 73% |
| Multi-step reaction with 2 steps 1: mit Hilfe von Rhizopus nigricans 2: CrO3; acetic acid View Scheme |

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 60℃; for 0.5h; | 65% |
| With N-(2-chloro-1,1,2-trifluoroethyl)diethylamine In acetonitrile | |
| With hydrogenchloride In methanol Heating; | |
| With hydrogenchloride | |
| With hydrogenchloride; acetic acid |
-
-
58-18-4
17-methyltestosterone

| Conditions | Yield |
|---|---|
| With Penicillium notatum KCH 904 In water; acetone at 27℃; for 312h; Enzymatic reaction; | A 65% B 31% |
-
-
58-18-4
17-methyltestosterone

| Conditions | Yield |
|---|---|
| Stage #1: 17-methyltestosterone With C19H26ClIrN3O(1+)*Cl(1-) In water; acetonitrile at 80℃; for 0.166667h; Green chemistry; Stage #2: With formic acid In water; acetonitrile at 80℃; for 2h; Green chemistry; | 63% |
| (i) LiAlH4, THF, (ii) aq. HCl, acetone; Multistep reaction; |
-
-
462-95-3
formaldehyde diethyl acetal

-
-
58-18-4
17-methyltestosterone

-
-
80126-38-1
17β-hydroxy-17α-methyl-6-methyleneandrost-4-en-3-one

| Conditions | Yield |
|---|---|
| With sodium acetate; trichlorophosphate In chloroform for 1h; Heating; | 51% |
| With sodium acetate; trichlorophosphate In chloroform |

| Conditions | Yield |
|---|---|
| With copper(II) nitrate In ethanol for 12h; Heating; | A 47% B 6% |

-
-
58-18-4
17-methyltestosterone

-
B
-
1807-02-9
11α,17β-Dihydroxy-17α-methylandrost-4-en-3-one

-
C
-
972-50-9
7α,17β-dihydroxy-17-methyl-4-androsten-3-one

| Conditions | Yield |
|---|---|
| With D-glucose In water; acetone at 25℃; Reagent/catalyst; Microbiological reaction; | A 19% B 17% C 45% |

-
-
58-18-4
17-methyltestosterone

-
C
-
1807-02-9
11α,17β-Dihydroxy-17α-methylandrost-4-en-3-one

-
D
-
13096-49-6
6β,17β-dihydroxy-17α-methylandrost-4-en-3-one

-
F
-
972-50-9
7α,17β-dihydroxy-17-methyl-4-androsten-3-one

| Conditions | Yield |
|---|---|
| With D-glucose In water; acetone at 25℃; Reagent/catalyst; Microbiological reaction; | A 9% B 29% C 5% D 34% E 4% F 6% |

-
-
58-18-4
17-methyltestosterone

-
B
-
1807-02-9
11α,17β-Dihydroxy-17α-methylandrost-4-en-3-one

-
C
-
13096-49-6
6β,17β-dihydroxy-17α-methylandrost-4-en-3-one

-
G
-
972-50-9
7α,17β-dihydroxy-17-methyl-4-androsten-3-one

| Conditions | Yield |
|---|---|
| With D-glucose In water; acetone at 25℃; Reagent/catalyst; Microbiological reaction; | A 31% B 20% C 7% D 7% E 4% F 5% G 16% |

-
-
58-18-4
17-methyltestosterone

-
A
-
302-76-1
17-methyl-estra-1,3,5(10)-triene-3,17α-diol

-
D
-
13096-49-6
6β,17β-dihydroxy-17α-methylandrost-4-en-3-one

-
E
-
33526-41-9
6β,17β-dihydroxy-17α-methyl-1,4-androstadien-3-one

| Conditions | Yield |
|---|---|
| With Acremonium strictum PTCC 5282 strain at 25℃; for 144h; pH=6.5; Enzymatic reaction; | A 7.6% B 24.3% C 16.7% D 28.4% E 9.5% |

-
-
58-18-4
17-methyltestosterone

-
A
-
13096-49-6
6β,17β-dihydroxy-17α-methylandrost-4-en-3-one

| Conditions | Yield |
|---|---|
| With fungus Cephalosporium aphidicola In ethanol for 120h; hydroxylation; | A 17% B 4% |

| Conditions | Yield |
|---|---|
| With liver microsomes from Aroclor 1254-induced male Wistar rat In methanol; phosphate buffer at 37℃; pH=7.4; | A 14% B n/a C n/a |

-
-
58-18-4
17-methyltestosterone

| Conditions | Yield |
|---|---|
| With silica gel for 46h; Irradiation; | 7.9% |

| Conditions | Yield |
|---|---|
| for 4h; Irradiation; | A 1% B 5% |
-
-
123-75-1
pyrrolidine

-
-
58-18-4
17-methyltestosterone

-
-
95908-76-2
17α-methyl-3-pyrrolidino-androsta-3,5-dien-17β-ol

| Conditions | Yield |
|---|---|
| With benzene |

| Conditions | Yield |
|---|---|
| With tetrahydrofuran; ammonia; lithium |

| Conditions | Yield |
|---|---|
| With copper(II) sulfate at 135 - 150℃; under 0.01 Torr; |
17-Methyltestosterone Chemical Properties
IUPAC Name: (8R,9S,10R,13S,14S,17S)-17-Hydroxy-10,13,17-trimethyl-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-3-one
Molecular Structure: 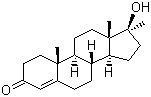
Molecular Formula : C20H30O2
Molecular Weight : 302.45
CAS NO: 58-18-4
EINECS : 200-366-3
Index of Refraction: 1.556
Surface Tension: 43.7 dyne/cm
Density: 1.1 g/cm3
Flash Point: 185.3 °C
Enthalpy of Vaporization: 79.71 kJ/mol
Boiling Point: 434.4 °C at 760 mmHg
Vapour Pressure: 2.28E-09 mmHg at 25 °C
Melting point 162-168 &dec;C
Alpha : 79 º (c=1, alcohol)
Storage temp: 2-8 °C
Solubility H2O: ≤0.5 mg/mL
Appearance: Methyltestosterone (CAS NO. 58-18-4) is white to slightly yellowish-white crystalline .
Classification Code of Methyltestosterone (CAS NO. 58-18-4): Anabolic Agents; Androgen; Antineoplastic Agents; Antineoplastic agents, hormonal; Drug / Therapeutic Agent; Hormone; Hormones; Hormones, Hormone Substitutes, and Hormone Antagonists; Human Data; Mutation data; Reproductive Effect; Tumor data
17-Methyltestosterone Uses
1.Methyltestosterone (CAS NO. 58-18-4) is used for androgen medicines, used for supplement treatment with testosterone deficiency .
2.Methyltestosterone (CAS NO. 58-18-4) can also be used for functional bleeding of uterine, aplastic anemia and other diseases treatment.
17-Methyltestosterone Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| man | TDLo | oral | 39mg/kg/33W-I (39mg/kg) | LIVER: "JAUNDICE, CHOLESTATIC" | American Journal of Gastroenterology. Vol. 82, Pg. 461, 1987. |
| mouse | LD50 | intraperitoneal | 400mg/kg (400mg/kg) | Drugs in Japan Vol. 6, Pg. 830, 1982. | |
| mouse | LD50 | oral | 1860mg/kg (1860mg/kg) | Drugs in Japan Vol. 6, Pg. 830, 1982. | |
| mouse | LD50 | subcutaneous | > 5gm/kg (5000mg/kg) | Drugs in Japan Vol. 6, Pg. 830, 1982. | |
| rat | LD50 | intraperitoneal | 1050mg/kg (1050mg/kg) | Drugs in Japan Vol. 6, Pg. 830, 1982. | |
| rat | LD50 | oral | 2500mg/kg (2500mg/kg) | Drugs in Japan Vol. 6, Pg. 830, 1982. | |
| rat | LD50 | subcutaneous | 5gm/kg (5000mg/kg) | Drugs in Japan Vol. 6, Pg. 830, 1982. |
17-Methyltestosterone Safety Profile
Hazard Codes:  T,
T, Xn,
Xn, Xi ,
Xi ,![]() F
F
Risk Statements: 45-63-22-20-19-11-61-60-36/37/38
R45:May cause cancer.
R63:Possible risk of harm to the unborn child.
R22:Harmful if swallowed.
R20:Harmful by inhalation.
R19:May form explosive peroxides.
R11:Highly flammable.
R60:May impair fertility.
R61:May cause harm to the unborn child.
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 53-36/37-45-24/25-22
S53:Avoid exposure - obtain special instructions before use.
S36/37:Wear suitable protective clothing and gloves.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S24/25:Avoid contact with skin and eyes.
S22:Do not breathe dust.
RIDADR: 3249
WGK Germany: 3
RTECS: BV8400000
HazardClass: 6.1(b)
PackingGroup: III
17-Methyltestosterone Specification
Methyltestosterone (CAS NO. 58-18-4), its Synonyms are (17-beta)-17-Hydroxy-17-methylandrost-4-en-3-one ; 17(alpha)-Methyl-delta4-androsten-17(beta)-ol-3-one ; 17-Hydroxy-17-methyl-3-keto-androstene-4 ; 17-beta-Hydroxy-17-methylandrost-4-en-3-one ; 17alpha-Methyl-3-oxo-4-androsten-17beta-ol ; 17alpha-Methyl-delta-androsten-17beta-ol-3-one ; M.T.Mucorettes ; Malestrone ; Androst-4-en-3-one, 17-beta-hydroxy-17-methyl- ; Androst-4-en-3-one, 17-hydroxy-17-methyl-, (17beta)- ; Androst-4-en-3-one, 17beta-hydroxy-17-methyl- .
Related Products
- 17-Methyltestosterone
- 58185-39-0
- 58185-47-0
- 58186-27-9
- 581-89-5
- 5819-04-5
- 58193-25-2
- 5819-40-9
- 58194-26-6
- 58-19-5
- 58196-33-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View