-
Name
2,2-Dimethylbutane
- EINECS 200-906-8
- CAS No. 75-83-2
- Article Data251
- CAS DataBase
- Density 0.649
- Solubility insoluble
- Melting Point -115 ºC
- Formula C6H14
- Boiling Point 50 ºC
- Molecular Weight 86.1772
- Flash Point -48 ºC
- Transport Information UN 1208
- Appearance Liquid.
- Safety Probably an irritant and narcotic in high concentration. A very dangerous fire and explosion hazard when exposed to heat or flame; can react vigorously with oxidizing materials. Keep away from heat or open flame. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes.
- Risk Codes R11;R38;R51/53;R65;R67
-
Molecular Structure
- Hazard Symbols F;Xn;N
- Synonyms 2,2-Dimethylbutane;3,3-Dimethylbutane; NSC 74126; Neohexane
- PSA 0.00000
- LogP 2.44250
Synthetic route

| Conditions | Yield |
|---|---|
| With nickel(II) bis(octanoate); hydrogen; 1,4-bis(2,6-diisopropylphenyl)-2,3-dimethyl-1,4-diazabuta-1,3-diene; 4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane In benzene-d6 at 50℃; under 3040.2 Torr; for 5h; | 98% |
| With C28H32PZr(1+)*C19H3BF15(1-); hydrogen at 20℃; for 2h; Reagent/catalyst; Glovebox; Schlenk technique; | 86% |
| With borane-ammonia complex; Pd(SIPr)(PCy3) In isopropyl alcohol at 50℃; for 16h; Inert atmosphere; Glovebox; | 69% |
-
-
558-37-2
tert-butylethylene

-
-
6872-06-6
2-methyl indoline

-
A
-
95-20-5
2-methyl-1H-indole

-
B
-
75-83-2
2,2-Dimethylbutane

| Conditions | Yield |
|---|---|
| With (PSCOP)IrHCl; sodium t-butanolate In para-xylene at 120℃; for 12h; Inert atmosphere; Schlenk technique; Glovebox; Sealed tube; | A 96% B n/a |


| Conditions | Yield |
|---|---|
| <(phen)PdCH3(Et2O)>+- In dichloromethane at 25℃; for 24h; Yields of byproduct given; | A n/a B 94% |


| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid In 1,1,2-Trichloro-1,2,2-trifluoroethane 1.) -30 deg C 2.) room temp.; | A 92.5% B 7.5% |


| Conditions | Yield |
|---|---|
| With hydrogenchloride; aluminium trichloride In dichloromethane at 20℃; for 1h; | 92% |
-
-
493-05-0
isochromane

-
-
558-37-2
tert-butylethylene

-
A
-
253-35-0
1H-isochromene

-
B
-
75-83-2
2,2-Dimethylbutane

| Conditions | Yield |
|---|---|
| With (PSCOP)IrHCl; sodium t-butanolate In para-xylene at 120℃; for 12h; Inert atmosphere; Schlenk technique; Glovebox; Sealed tube; | A 91% B n/a |


| Conditions | Yield |
|---|---|
| With hydrogen; K-10 montmorillonite; platinum In diethylene glycol dimethyl ether under 37503 Torr; for 20h; Reduction; | 60% |
| With hydrogen; aluminum oxide; nickel at 190℃; | 46% |
| With ethanol; sulfuric acid at 50℃; Electrolysis.elektrolytische Reduktion an einer Cadmiumkathode; | |
| With molybdenum (IV) sulfide Hydrogenation.unter hohem Druck; | |
| Multi-step reaction with 2 steps 1: water; alcohol; hydrazine hydrate 2: potassium hydroxide; platinized fired clay / 180 °C View Scheme |
-
-
96-47-9
2-methyltetrahydrofuran

-
-
558-37-2
tert-butylethylene

-
A
-
534-22-5
2-methylfuran

-
B
-
75-83-2
2,2-Dimethylbutane

| Conditions | Yield |
|---|---|
| With (PSCOP)IrHCl; sodium t-butanolate In para-xylene at 150℃; for 12h; Inert atmosphere; Schlenk technique; Glovebox; Sealed tube; | A 57% B n/a |

-
-
1125-99-1
1-(1-Cyclohexen-1-yl)pyrrolidine

-
-
558-37-2
tert-butylethylene

-
A
-
635-90-5
1-phenylpyrrole

-
B
-
75-83-2
2,2-Dimethylbutane

-
C
-
62672-96-2
N-(1-cyclohexen-1-yl)-1H-pyrrole

| Conditions | Yield |
|---|---|
| With (PSCOP)IrHCl; sodium t-butanolate In para-xylene at 150℃; for 24h; Inert atmosphere; Schlenk technique; Glovebox; Sealed tube; | A 16% B n/a C 54% |

-
-
496-16-2
2,3-Dihydrobenzofuran

-
-
558-37-2
tert-butylethylene

-
A
-
271-89-6
1-benzofurane

-
B
-
75-83-2
2,2-Dimethylbutane

-
C
-
41014-29-3
2,2'-bibenzofuranyl

| Conditions | Yield |
|---|---|
| With (PSCOP)IrHCl; sodium t-butanolate In para-xylene at 120℃; for 6h; Inert atmosphere; Schlenk technique; Glovebox; Sealed tube; | A 11% B n/a C 37% |

-
-
110-54-3
hexane

-
A
-
96-14-0
3-methylpentane

-
B
-
107-83-5
2-Methylpentane

-
C
-
75-83-2
2,2-Dimethylbutane

-
D
-
79-29-8
2,3-dimethylbutane

| Conditions | Yield |
|---|---|
| platinum at 250℃; Product distribution; Further Variations:; Catalysts; Temperatures; | A 22.1% B 34.8% C 12.9% D 9% |
| With hydrogen at 215℃; under 7500.75 Torr; Catalytic behavior; Kinetics; Reagent/catalyst; Temperature; Flow reactor; Overall yield = 62.1 %; | A n/a B n/a C 6.7% D 8% |
| Pt-Al2O3-Cl at 100 - 140℃; under 15001.2 Torr; Product distribution; |


| Conditions | Yield |
|---|---|
| at 200℃; for 1h; Catalytic behavior; Kinetics; Temperature; Time; | A n/a B 16% |
| aluminum oxide; {4-Me2N-C6H2-2,6-[OP(t-Bu)2]2}IrH2 at 125℃; for 0.166667 - 4h; Product distribution / selectivity; | |
| aluminum oxide; {4-Me2N-C6H2-2,6-[OP(t-Bu)2]2}IrH2 at 125℃; for 0.25 - 4h; Product distribution / selectivity; | |
| {4-Me2N-C6H2-2,6-[OP(t-Bu)2]2}IrH2 at 125℃; for 0.0833333 - 4h; Product distribution / selectivity; | |
| With (PSCOP)IrHCl; sodium t-butanolate at 100℃; for 1h; Inert atmosphere; Schlenk technique; Glovebox; Sealed tube; regioselective reaction; |

-
-
34557-54-5
methane

-
A
-
562-49-2
3,3-dimethylpentane

-
B
-
590-73-8
2,2-dimethylhexane

-
C
-
594-82-1
tetramethyl-2,2,3,3 butane

-
D
-
1067-08-9
3-ethyl-3-methyl-pentane

-
E
-
1067-20-5
tetraethylmethane

-
F
-
13475-81-5
2,2,3,3-tetramethyl-hexane

-
G
-
589-43-5
2,4-dimethylhexane

-
H
-
74-84-0
ethane

-
I
-
74-98-6
propane

-
J
-
75-28-5
Isobutane

-
K
-
463-82-1
2,2-dimethylpropane

-
L
-
78-78-4
methylbutane

-
M
-
107-83-5
2-Methylpentane

-
N
-
75-83-2
2,2-Dimethylbutane

-
O
-
464-06-2
triptane

-
P
-
590-35-2
2,2-dimethylpentane

-
Q
-
7154-79-2
2,2,3,3-tetramethylpentane

-
R
-
1333-74-0
hydrogen

| Conditions | Yield |
|---|---|
| With water at 84℃; Product distribution / selectivity; Photolysis; | A 1.89% B 1.45% C 9.67% D 1.72% E 5.82% F 2.18% G 1.45% H 0.17% I 6.34% J 4.51% K 15.7% L 4.01% M 0.4% N 6.41% O 3.52% P 1.2% Q 9.66% R 3.44% |
| at 84℃; Product distribution / selectivity; Photolysis; 24 psig; | A 2.26% B 1.83% C 7.61% D 1.75% E 9.61% F 1.56% G 1.82% H 0.035% I 7.34% J 5.28% K 12.2% L 2.1% M 0.97% N 7.04% O 3.05% P 2.35% Q 6.46% R 0.219% |


| Conditions | Yield |
|---|---|
| antimony pentafluoride; zirconium(IV) oxide at 0℃; under 50 Torr; Product distribution; also 2-methylbutane, var conditions, var. catalysts; | A 12.6% B 10.1% C 0.1% |


| Conditions | Yield |
|---|---|
| With hydrogen; tungsten-zirconia-platinum catayst In water at 287.768℃; Product distribution / selectivity; | A 9.377% B 7.065% |
| With hydrogen at 220℃; under 20 Torr; |
-
-
78-78-4
methylbutane

-
-
74-85-1
ethene

-
A
-
591-76-4
2-Methylhexane

-
B
-
565-59-3
2,3-dimethyl pentane

-
C
-
96-14-0
3-methylpentane

-
D
-
107-83-5
2-Methylpentane

-
E
-
75-83-2
2,2-Dimethylbutane

-
F
-
79-29-8
2,3-dimethylbutane

| Conditions | Yield |
|---|---|
| water; fluorosulphonic acid at -15℃; for 1.5h; Product distribution; | A 5.8% B 6.2% C 8% D n/a E 6.5% F n/a |

-
-
464-06-2
triptane

-
A
-
463-82-1
2,2-dimethylpropane

-
B
-
75-83-2
2,2-Dimethylbutane

-
C
-
79-29-8
2,3-dimethylbutane

| Conditions | Yield |
|---|---|
| nickel at 231.9℃; under 41.5 Torr; for 0.0833333h; Product distribution; Mechanism; hydrogenolysis; | A 1.6% B 1% C 0.031% D n/a |

-
-
110-54-3
hexane

-
A
-
78-78-4
methylbutane

-
B
-
96-14-0
3-methylpentane

-
C
-
107-83-5
2-Methylpentane

-
D
-
75-83-2
2,2-Dimethylbutane

-
E
-
79-29-8
2,3-dimethylbutane

-
F
-
109-66-0
pentane

| Conditions | Yield |
|---|---|
| With hydrogen at 180℃; under 11251.1 Torr; for 0.5h; | A 0.6% B n/a C n/a D n/a E n/a F 0.3% |

-
-
56-23-5
tetrachloromethane

-
-
507-20-0
tertiary butyl chloride

-
-
557-20-0
diethylzinc

-
-
75-83-2
2,2-Dimethylbutane


| Conditions | Yield |
|---|---|
| With nickel; copper at 250℃; Hydrogenation; | |
| With nickel-copper at 250℃; Hydrogenation; |

| Conditions | Yield |
|---|---|
| With dibutyl ether at 50℃; |
-
-
75-83-2
2,2-Dimethylbutane

| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: CH4; heating of mixt. of Zr(Me)(NHCMe3)((C9H10)2CH2CH2) and CH3CH2CMe3 at 75°C for 24 h; | 98% |

| Conditions | Yield |
|---|---|
| In hexane at -15℃; | 77% |
-
-
75-83-2
2,2-Dimethylbutane

-
-
79-31-2
isobutyric Acid

-
-
14253-02-2
trimethylolpropane tri(2-methylpropanoate)

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene at 120℃; for 6h; | 72% |

| Conditions | Yield |
|---|---|
| With Rh2[R-tris(p-tBuC6H4)TPCP]4 In dichloromethane Reflux; enantioselective reaction; | 70% |
-
-
75-83-2
2,2-Dimethylbutane

| Conditions | Yield |
|---|---|
| With B(C6F5)3 In further solvent(s) byproducts: CH4; (Ar); std. drybox technique; tert-butylethane was added to mixt. of Pt complex and B(C6F5)3 (1 equiv.); mixt. was stirred at 35°C for 3 d; solvent removed (vac.); chromd. (alumina, CH2Cl2); recrystd. (CH2Cl2/methanol, -30°C); | 65% |
-
-
75-83-2
2,2-Dimethylbutane

-
-
82721-80-0
1-methyl-4-((phenylsulfonyl)ethynyl)benzene

| Conditions | Yield |
|---|---|
| With pyridine-4-carbonitrile; 4-cyanopyridine N-oxide; 2-(5,5-dimethyl-1,3,2-dioxaborinan-2-yl)-5,5-dimethyl-1,3,2-dioxaborinane at 50℃; for 12h; Inert atmosphere; Schlenk technique; | 64% |
-
-
75-83-2
2,2-Dimethylbutane

-
-
164400-22-0
Cp(*)W(NO)(η(2)-CPhCH2)(CH2Si(CH3)3)

| Conditions | Yield |
|---|---|
| In neat (no solvent) N2 or Ar-atmosphere; 54°C (24 h), evapn. (vac., 1 h); Et2O addn., filtering, concg. (vac.), crystn. (-30°C, 24 h); elem. anal.; | 59% |

| Conditions | Yield |
|---|---|
| With [IPr2*NN]Cu(η2-C6H6) In fluorobenzene at 20℃; Inert atmosphere; Glovebox; Sealed tube; | A 4% B 53% |

-
-
75-83-2
2,2-Dimethylbutane

-
-
103-71-9
phenyl isocyanate

-
A
-
1361973-98-9
2-{2-(3,3-dimethyl)butyl}benzoxazole

| Conditions | Yield |
|---|---|
| With di-tert-butyl peroxide; copper diacetate In acetonitrile at 120℃; for 10h; Inert atmosphere; Sealed tube; regioselective reaction; | A 50% B 13% C 18% |


| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene In dichloromethane at 20℃; for 2h; | 45% |
-
-
75-83-2
2,2-Dimethylbutane

-
A
-
354-92-7
perfluoroisobutylene

-
B
-
354-96-1
perfluoro(2,3-dimethylbutane)

-
C
-
355-04-4
perfluoroisohexane

-
D
-
865-71-4
perfluoro(3-methylpentane)

-
E
-
1805-22-7
perfluoro(2-methylcyclopentane)

-
F
-
112156-74-8
perfluoro(2,2-dimethylbutane)

| Conditions | Yield |
|---|---|
| cobalt (III) fluoride at 360℃; for 3h; Product distribution; | A 7% B 4% C 18% D 16% E 12% F 43% |

-
-
75-83-2
2,2-Dimethylbutane

-
-
646535-41-3
[platinum(IV)(trimethyl)(((4-tert-butyl-2,6-dimethylphenyl)NC(CH3))2CH)]

-
-
646535-49-1
[Pt(CH(C(CH3)NC6H2(CH3)2C(CH3)3)2)(H)(CH2CHC(CH3)3)]

| Conditions | Yield |
|---|---|
| In further solvent(s) heating in neohexane at 35°C for 110-200 h; | 40% |
2,2-DIMETHYLBUTANE Chemical Properties
The Molar mass of 2,2-DIMETHYLBUTANE(75-83-2): 86.17 g/mol
EINECS: 200-906-8
Density: 0.791 g/l, liquid
Melting point: -98.8 °C, 174 K, -146 °F
Boiling point: 49.73 °C, 323 K, 122 °F
Flash point: -29 °C (closed cup)
Solubility in water: Insoluble
vapor density: 2.97 (vs air)
vapor pressure: 5.35 psi ( 20 °C)
refractive index: n20/D 1.369
Appearance: colorless liquid
storage temp.: Flammables area
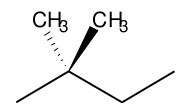
2,2-Dimethylbutane(75-83-2), trivially known as neohexane, is the isomer of hexane containing a quaternary carbon.It is a hydrocarbon, and it has covalent bonds and is single bonded.
The Structure of 2,2-DIMETHYLBUTANE(75-83-2):
2,2-DIMETHYLBUTANE Uses
2,2-DIMETHYLBUTANE(75-83-2) is also used in organic synthesis and contrast samples for gas chromatography.
2,2-DIMETHYLBUTANE Toxicity Data With Reference
2,2-DIMETHYLBUTANE Consensus Reports
2,2-DIMETHYLBUTANE Safety Profile
Hazard Codes:
 F,
F, Xn,
Xn, N
N Risk Statements: 11-38-51/53-65-67
11: Highly Flammable
38: Irritating to the skin
51/53: Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment
65: Harmful: May cause lung damage if swallowed
67: Vapors may cause drowsiness and dizziness
Safety Statements: 9-16-29-33-61-62
9: Keep container in a well-ventilated place
16: Keep away from sources of ignition - No smoking
29: Do not empty into drains
33: Take precautionary measures against static discharges
61: Avoid release to the environment. Refer to special instructions safety data sheet
62: If swallowed, do not induce vomiting: seek medical advice immediately and show this container or label
RIDADR: UN 1208 3/PG 2
WGK Germany: 3
HazardClass: 3
PackingGroup: II
2,2-DIMETHYLBUTANE Standards and Recommendations
ACGIH TLV: TWA 500 ppm; STEL 1000 ppm
DFG MAK: 200 ppm (720 mg/m3)
NIOSH REL: (Alkanes) TWA 350 mg/m3
Related Products
- 20,22-DIHYDRODIGITOXIN
- 20,29,30-Trinorlupane,(17alpha)-
- 20-ETHYL-6-β,8-DIHYDROXY-1-α-METHOXY-4-METHYLHETERATISAN-14-ONE
- 20-Ethylprostaglandin F2-alpha
- 20-Isopropylcholanthrene
- 20-METHYLCHOLANTHREN-15-ONE
- 20-METHYLCHOLANTHRENE PICRATE
- 20-METHYLCHOLANTHRENE-TRINITRO-BENZENE
- 20(S)-Ginsenoside C-K
- 2,10-DIFLUOROBENZO(rst)PENTAPHENE
- 7583-27-9
- 75833-38-4
- 7583-53-1
- 7583-60-0
- 75838-07-2
- 75838-24-3
- 7584-05-6
- 7584-11-4
- 758-42-9
- 75-84-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View