-
Name
2,3-Dimethylbutane
- EINECS 201-193-6
- CAS No. 79-29-8
- Article Data314
- CAS DataBase
- Density 0.662 g/mL at 25 °C(lit.)
- Solubility
- Melting Point -129 °C
- Formula C6H14
- Boiling Point 58 °C(lit.)
- Molecular Weight 86.1772
- Flash Point ?28 °F
- Transport Information UN 2457 3
- Appearance clear, colorless liquid
- Safety Probably an irritant and narcotic in high concentration. A very dangerous fire and explosion hazard when exposed to heat or flame; can react vigorously with oxidizing materials. Keep away from heat and open flame. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes.
- Risk Codes 11-65
-
Molecular Structure
- Hazard Symbols Flammable, dangerous fire and explosion risk.
- Synonyms 1,1,2,2-Tetramethylethane;2,3-Dimethylbutane; Biisopropyl; Diisopropyl; NSC 24837
- PSA 0.00000
- LogP 2.29840
Synthetic route

| Conditions | Yield |
|---|---|
| With C40H56N2RuSi4; hydrogen In toluene at 25℃; under 760.051 Torr; for 6h; Schlenk technique; | 99% |
| With C40H56FeN2Si4(2-); hydrogen In 1,2-dimethoxyethane at 80℃; under 7600.51 Torr; for 6h; Schlenk technique; Autoclave; | 99% |
| With nickel(II) bis(octanoate); hydrogen; 1,4-bis(2,6-diisopropylphenyl)-2,3-dimethyl-1,4-diazabuta-1,3-diene; 4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane In benzene-d6 at 50℃; under 3040.2 Torr; for 24h; Reagent/catalyst; | 91% |

| Conditions | Yield |
|---|---|
| With C28H32PZr(1+)*C19H3BF15(1-); hydrogen at 20℃; for 2h; Reagent/catalyst; Glovebox; Schlenk technique; | 89% |
| at 82℃; Hydrogenation; | |
| With nickel kieselguhr under 73550.8 Torr; Hydrogenation; |
-
-
12176-06-6
hydrido(tricarbonyl)(cyclopentadienyl)molybdenum

-
-
1493-13-6
trifluorormethanesulfonic acid

-
-
563-78-0
2,3-Dimethyl-1-butene

-
B
-
79-29-8
2,3-dimethylbutane

| Conditions | Yield |
|---|---|
| In dichloromethane-d2 react. at -75°C for 5 min; quenching by pyridine, detecting by n.m.r. spectroscopy; | A n/a B 86% |

-
-
87050-44-0
(η5-pentamethylcyclopentadienyl)hafnium(2,3-dimethyl-1,3-butadiene)chloride

-
-
1333-74-0
hydrogen

-
A
-
563-79-1
2,3-Dimethyl-2-butene

-
B
-
113180-42-0
tetrakis{(pentamethylcyclopentadienyl)hafnium dihydride chloride}*(toluene)0.5

-
C
-
79-29-8
2,3-dimethylbutane

| Conditions | Yield |
|---|---|
| In toluene A pressure vessel is filled with a soln. of (C5Me5)Hf(C6H10)Cl in toluene and charged with 10 atm of H2, reaction mixture is stirred overnight at 70°C under N2.; Excess H2 is released, hot soln. is transferred into a Schlenk vessel, cooling of the soln. to room temp., a bright yellow ppt. is formed, solvent is decanted, ppt. is redissolved (hot toluene), slow cooling to -30°C, elem. anal.; | A n/a B 56% C n/a |

-
-
513-81-5
2,3-dimethyl-buta-1,3-diene

-
A
-
563-79-1
2,3-Dimethyl-2-butene

-
B
-
79-29-8
2,3-dimethylbutane

-
C
-
563-78-0
2,3-Dimethyl-1-butene

| Conditions | Yield |
|---|---|
| With hydrogen; palladium dichloride In N,N-dimethyl-formamide under 18751.5 Torr; for 0.25h; Product distribution; Ambient temperature; various time; | A 39.3% B 0.05% C 52.2% |
| With hydrogen; 1,5-hexadienerhodium(I)-chloride dimer In various solvent(s) for 2h; Ambient temperature; pH=7.6; | A 27% B 25% C 22% |
| With bis(1,5-cyclooctadiene)rhodium(I) tetrafluoroborate; hydrogen; disodium salt of the bis(m-sulfonatophenyl)-n-butylphosphane at 60℃; under 22502.3 Torr; for 6h; Ionic liquid; chemoselective reaction; |

-
-
1418118-69-0
C30H74O6Si6

-
A
-
110-54-3
hexane

-
B
-
96-14-0
3-methylpentane

-
C
-
107-83-5
2-Methylpentane

-
D
-
79-29-8
2,3-dimethylbutane

| Conditions | Yield |
|---|---|
| With H2SiEt2; [(2,6-[OP(tBu)2]2C6H3)Ir(H)(acetone)]+[B(C6F5)4]- for 336h; Inert atmosphere; Glovebox; | A 45% B 6% C 8% D n/a |

-
-
1516-68-3
O,O-diethyl azidophosphate

-
A
-
1068-21-9
Diethyl phosphoramidate

-
B
-
79-29-8
2,3-dimethylbutane

-
C
-
123150-70-9
(1,1,2-Trimethyl-propyl)-phosphoramidic acid diethyl ester

-
D
-
123150-71-0
(2,3-Dimethyl-butyl)-phosphoramidic acid diethyl ester

| Conditions | Yield |
|---|---|
| In various solvent(s) at 22℃; Irradiation; | A 40.1% B n/a C 28.2% D 30.6% |

-
-
67-56-1
methanol

-
-
563-79-1
2,3-Dimethyl-2-butene

-
A
-
79-29-8
2,3-dimethylbutane

-
B
-
55505-23-2
2,2,3-trimethyl-1-butanol

-
C
-
52670-36-7
hexamethyl-2,3,3,4,4,5 hexane

| Conditions | Yield |
|---|---|
| With europium(III) chloride In methanol for 8h; Irradiation; | A 26% B 37% C 4% |

-
-
110-54-3
hexane

-
A
-
96-14-0
3-methylpentane

-
B
-
107-83-5
2-Methylpentane

-
C
-
75-83-2
2,2-Dimethylbutane

-
D
-
79-29-8
2,3-dimethylbutane

| Conditions | Yield |
|---|---|
| platinum at 250℃; Product distribution; Further Variations:; Catalysts; Temperatures; | A 22.1% B 34.8% C 12.9% D 9% |
| With hydrogen at 215℃; under 7500.75 Torr; Catalytic behavior; Kinetics; Reagent/catalyst; Temperature; Flow reactor; Overall yield = 62.1 %; | A n/a B n/a C 6.7% D 8% |
| Pt-Al2O3-Cl at 100 - 140℃; under 15001.2 Torr; Product distribution; |

-
-
1418118-67-8
C22H52O5Si4

-
A
-
110-54-3
hexane

-
B
-
96-14-0
3-methylpentane

-
C
-
107-83-5
2-Methylpentane

-
D
-
79-29-8
2,3-dimethylbutane

| Conditions | Yield |
|---|---|
| With H2SiEt2; [(2,6-[OP(tBu)2]2C6H3)Ir(H)(acetone)]+[B(C6F5)4]- for 336h; Inert atmosphere; Glovebox; | A 17% B 32% C 31% D n/a |

-
-
67-56-1
methanol

-
A
-
108-08-7
2,4-dimethylpentane

-
B
-
565-59-3
2,3-dimethyl pentane

-
C
-
75-28-5
Isobutane

-
D
-
78-78-4
methylbutane

-
E
-
96-14-0
3-methylpentane

-
F
-
107-83-5
2-Methylpentane

-
G
-
79-29-8
2,3-dimethylbutane

-
H
-
464-06-2
triptane

-
I
-
95-93-2
1,2,4,5-tetramethylbenzene

-
J
-
527-53-7
1,2,3,5-Tetramethylbenzene

-
K
-
700-12-9
pentamethylbenzene,

-
L
-
87-85-4
Hexamethylbenzene

| Conditions | Yield |
|---|---|
| With isopropyl alcohol; indium (III) iodide at 200℃; for 2 - 3h; | A n/a B n/a C n/a D n/a E n/a F n/a G n/a H 16.7% I n/a J n/a K n/a L n/a |

-
-
67-56-1
methanol

-
A
-
75-28-5
Isobutane

-
B
-
78-78-4
methylbutane

-
C
-
96-14-0
3-methylpentane

-
D
-
107-83-5
2-Methylpentane

-
E
-
79-29-8
2,3-dimethylbutane

-
F
-
464-06-2
triptane

-
G
-
700-12-9
pentamethylbenzene,

-
H
-
87-85-4
Hexamethylbenzene

| Conditions | Yield |
|---|---|
| With isopropyl alcohol; indium (III) iodide at 200℃; for 2 - 3h; Product distribution / selectivity; | A 7.5% B 8.7% C 0.9% D 1.4% E 2.9% F 12.8% G 7.1% H 3.1% |
| With hypophosphorous acid; isopropyl alcohol; indium (III) iodide at 200℃; for 2 - 3h; Product distribution / selectivity; | A 10.7% B 10.5% C 1.5% D 2.4% E 2.2% F 9.8% G 3.6% H 1.3% |


| Conditions | Yield |
|---|---|
| With hydrogen; tungsten-zirconia-platinum catayst In water at 287.768℃; Product distribution / selectivity; | A 9.377% B 7.065% |
| With hydrogen at 220℃; under 20 Torr; |
-
-
78-78-4
methylbutane

-
-
74-85-1
ethene

-
A
-
591-76-4
2-Methylhexane

-
B
-
565-59-3
2,3-dimethyl pentane

-
C
-
96-14-0
3-methylpentane

-
D
-
107-83-5
2-Methylpentane

-
E
-
75-83-2
2,2-Dimethylbutane

-
F
-
79-29-8
2,3-dimethylbutane

| Conditions | Yield |
|---|---|
| water; fluorosulphonic acid at -15℃; for 1.5h; Product distribution; | A 5.8% B 6.2% C 8% D n/a E 6.5% F n/a |

-
-
75-28-5
Isobutane

-
-
74-85-1
ethene

-
A
-
560-21-4
2,3,3-Trimethyl-pentane

-
B
-
78-78-4
methylbutane

-
C
-
107-83-5
2-Methylpentane

-
D
-
79-29-8
2,3-dimethylbutane

| Conditions | Yield |
|---|---|
| aluminium trichloride-diethyl ether (1/1) at 30℃; under 1520 Torr; for 0.5h; Further byproducts given; | A n/a B 5.2% C n/a D n/a |

-
-
75-28-5
Isobutane

-
-
74-85-1
ethene

-
A
-
565-75-3
2,3,4-trimethylpentane

-
B
-
78-78-4
methylbutane

-
C
-
107-83-5
2-Methylpentane

-
D
-
79-29-8
2,3-dimethylbutane

| Conditions | Yield |
|---|---|
| aluminium trichloride-diethyl ether (1/1) at 30℃; under 1520 Torr; for 0.5h; Further byproducts given; | A 3.6% B 5.2% C n/a D n/a |

-
-
75-28-5
Isobutane

-
-
74-85-1
ethene

-
A
-
584-94-1, 116724-01-7
2,3-dimethylhexane

-
B
-
78-78-4
methylbutane

-
C
-
107-83-5
2-Methylpentane

-
D
-
79-29-8
2,3-dimethylbutane

| Conditions | Yield |
|---|---|
| aluminium trichloride-diethyl ether (1/1) at 30℃; under 1520 Torr; for 0.5h; Further byproducts given; | A n/a B 5.2% C n/a D n/a |

-
-
75-28-5
Isobutane

-
-
74-85-1
ethene

-
A
-
589-43-5
2,4-dimethylhexane

-
B
-
78-78-4
methylbutane

-
C
-
107-83-5
2-Methylpentane

-
D
-
79-29-8
2,3-dimethylbutane

| Conditions | Yield |
|---|---|
| aluminium trichloride-diethyl ether (1/1) at 30℃; under 1520 Torr; for 0.5h; Further byproducts given; | A 2.5% B 5.2% C n/a D n/a |

-
-
67-56-1
methanol

-
-
75-28-5
Isobutane

-
A
-
78-78-4
methylbutane

-
B
-
79-29-8
2,3-dimethylbutane

-
C
-
464-06-2
triptane

-
D
-
700-12-9
pentamethylbenzene,

-
E
-
87-85-4
Hexamethylbenzene

| Conditions | Yield |
|---|---|
| With adamantane; indium (III) iodide at 200℃; for 12h; Product distribution / selectivity; | A n/a B n/a C 5% D n/a E n/a |

-
-
67-56-1
methanol

-
-
75-28-5
Isobutane

-
A
-
78-78-4
methylbutane

-
B
-
79-29-8
2,3-dimethylbutane

-
C
-
464-06-2
triptane

| Conditions | Yield |
|---|---|
| With adamantane; indium (III) iodide at 200℃; for 1 - 5h; Product distribution / selectivity; | A n/a B n/a C 4% |

-
-
464-06-2
triptane

-
A
-
463-82-1
2,2-dimethylpropane

-
B
-
75-83-2
2,2-Dimethylbutane

-
C
-
79-29-8
2,3-dimethylbutane

| Conditions | Yield |
|---|---|
| nickel at 231.9℃; under 41.5 Torr; for 0.0833333h; Product distribution; Mechanism; hydrogenolysis; | A 1.6% B 1% C 0.031% D n/a |

-
-
187737-37-7
propene

-
A
-
106-98-9
1-butylene

-
B
-
74-84-0
ethane

-
C
-
75-28-5
Isobutane

-
D
-
79-29-8
2,3-dimethylbutane

-
E
-
691-37-2
4-Methyl-1-pentene

-
F
-
592-42-7
1,5-Hexadien

| Conditions | Yield |
|---|---|
| at -78.1℃; Product distribution; excited by the impact of low-energy electrons; | A 0.92% B 0.12% C 0.92% D 0.43% E 1.12% F 0.24% |

-
-
110-54-3
hexane

-
A
-
78-78-4
methylbutane

-
B
-
96-14-0
3-methylpentane

-
C
-
107-83-5
2-Methylpentane

-
D
-
75-83-2
2,2-Dimethylbutane

-
E
-
79-29-8
2,3-dimethylbutane

-
F
-
109-66-0
pentane

| Conditions | Yield |
|---|---|
| With hydrogen at 180℃; under 11251.1 Torr; for 0.5h; | A 0.6% B n/a C n/a D n/a E n/a F 0.3% |


| Conditions | Yield |
|---|---|
| With tributylphosphine; copper In tetrahydrofuran at 25℃; for 0.0166667h; further 2-bromopropane, other temperatures; further t-butyl halide without P(n-Bu)3, other temperatures and times; | 41 % Chromat. |
| With diethyl ether; sodium | |
| With tributylphosphine; copper In tetrahydrofuran at 25℃; for 0.0166667h; | 41 % Chromat. |
| With Co(II)-bpy (2,2'-bipyridine); silver nitrate In acetonitrile at 25℃; Kinetics; Further Variations:; Reagents; Electrochemical reaction; |
-
-
75-44-5
phosgene

-
-
60-29-7
diethyl ether

-
-
3395-70-8
1-((benzyloxy)methyl)-3-chlorobenzene

-
-
920-39-8
isopropylmagnesium bromide

-
A
-
79-29-8
2,3-dimethylbutane

-
B
-
873-63-2
m-chlorobenzyl alcohol

-
C
-
108-88-3
toluene

-
D
-
100-51-6
benzyl alcohol


| Conditions | Yield |
|---|---|
| With molybdenum (IV) sulfide; xylene at 350℃; Hydrogenation.unter Druck; |

| Conditions | Yield |
|---|---|
| In gas reaction in a mass spectrometer; total pressure: 4E-6 Torr; | A 100% B 100% |

-
-
79-29-8
2,3-dimethylbutane

-
-
17640-15-2
methyl cyanoformate

-
A
-
26154-43-8
2,2,3-trimethyl-butyronitrile

| Conditions | Yield |
|---|---|
| tris(tetrabutylammonium)12-tungstophosphate In acetonitrile at 16℃; for 22h; Irradiation; Yields of byproduct given; | A n/a B 99% |
| sodium decatungstate In acetonitrile at 8℃; for 90h; Irradiation; | A 78% B 6% |


| Conditions | Yield |
|---|---|
| With methyltrifluoromethyldioxirane In dichloromethane at -22℃; for 0.05h; | 98% |
| With [PPh4]2[MnV(N)(CN)4]; dihydrogen peroxide; acetic acid In 2,2,2-trifluoroethanol at 23℃; for 5h; Reagent/catalyst; Inert atmosphere; | 96% |
| With [PPh4]2[MnV(N)(CN)4]; tetrabutylammonium periodite; acetic acid In 2,2,2-trifluoroethanol at 23℃; Inert atmosphere; | 95% |
-
-
79-29-8
2,3-dimethylbutane

-
A
-
594-57-0
2-chloro-2,3-dimethylbutane

-
B
-
600-06-6
1-chloro-2,3-dimethylbutane

| Conditions | Yield |
|---|---|
| With Perbenzoic acid; isocyanate de chlorosulfonyle Product distribution; Mechanism; other radical initiator or photochemically, selectivity of hydrogen abstraction as a function of conversion and temperature; | A 92.3% B 4.6% |
| bei der Chlorierung; | |
| With 4-tButyl iodobenzenedichloride; 3-(Dichloriodo)benzotrifluorid; (Dichloroiodo)benzene; 3-nitro(dichloroiodo)benzene In tetrachloromethane at 30℃; Product distribution; Competition reaction of reagents with cyclohexane. Relative rate constants.; |

| Conditions | Yield |
|---|---|
| With methanol; cerium(III) chloride; tetrabutyl-ammonium chloride In acetonitrile at 20℃; for 11h; Irradiation; Inert atmosphere; Sealed tube; | 81% |
-
-
79-29-8
2,3-dimethylbutane

-
-
52843-77-3
phenylethynyl trifluoromethyl sulfone

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile) for 10h; Heating; | 78% |

| Conditions | Yield |
|---|---|
| In acetonitrile Quantum yield; Thermodynamic data; Irradiation; ΔG; | A 70% B 7% |
| In acetonitrile Irradiation; | A 70% B 7% |
| In acetonitrile Quantum yield; Irradiation; var. solv.; | |
| In acetonitrile for 1h; Irradiation; Yield given. Yields of byproduct given. Title compound not separated from byproducts; |

-
-
79-29-8
2,3-dimethylbutane

-
-
210489-89-7
2-Trifluoromethanesulfonylmethyl-acrylic acid methyl ester

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile) for 14h; Heating; | 70% |

| Conditions | Yield |
|---|---|
| With 9,10-phenanthrenequinone In acetonitrile at 20℃; for 48h; UV-irradiation; Inert atmosphere; regioselective reaction; | 70% |

| Conditions | Yield |
|---|---|
| With {HB[3,5-(CF3)2Pz]3}Ag(THF) at 20℃; chemoselective reaction; | 69% |
-
-
79-29-8
2,3-dimethylbutane

-
-
119987-21-2
(E)-methyl 2-diazo-4-phenylbut-3-enoate

-
-
1130233-05-4
C17H24O2

| Conditions | Yield |
|---|---|
| With {HB[3,5-(CF3)2Pz]3}Ag(THF) at 20℃; chemoselective reaction; | 66% |
-
-
79-29-8
2,3-dimethylbutane

-
A
-
72221-03-5
2,3-dimethyl-1,2-epoxybutane

-
B
-
594-60-5
2,3-dimethylbutan-2-ol

| Conditions | Yield |
|---|---|
| With O(3P) at -80℃; Product distribution; | A 34% B 64% C n/a |

-
-
79-29-8
2,3-dimethylbutane

-
-
1048692-95-0
2-azido-4,4,4,5-tetramethyl-1,3,2-dioxaborolane

-
A
-
1048692-99-4
(HO)2BNH-cy5

| Conditions | Yield |
|---|---|
| In further solvent(s) byproducts: N2; Irradiation (UV/VIS); photolysis in quartz tube for 16 h (λ = 254 nm), 2,3-dimethylbutane-soln. of azide, room temp., Ar atm.; filtered, the solvent was removed under reduced pressure, sublimation (70°C, oil pump); | A n/a B 11% C 63% |


| Conditions | Yield |
|---|---|
| With 9-(2-mesityl)-10-methylacridinium perchlorate; 1,2-dibromomethane at 50℃; for 24h; Irradiation; | 63% |

| Conditions | Yield |
|---|---|
| With potassium phosphate; tetrakis(tetrabutylammonium)decatungstate(VI); [nickel(II) (4,4'-di-tert-butyl-2,2'-bipyridine)(bromide)2] In acetone at 30℃; for 18h; Inert atmosphere; Sealed tube; Irradiation; regioselective reaction; | 62% |

-
-
80146-01-6
pentamethylcyclopentadienyliridium(III) PMe3 dihydride

-
-
79-29-8
2,3-dimethylbutane

| Conditions | Yield |
|---|---|
| In further solvent(s) Irradiation (UV/VIS); soln. of complex in 2,3-dimethylbutane was irradiated for 36 h, then heated at 115°C for 21 h (inert atm.); cold chromy.(-80°C) on alumina III column, Et2O-hexane; elem. anal.; | 60% |
-
-
623-73-4
diazoacetic acid ethyl ester

-
-
79-29-8
2,3-dimethylbutane

-
-
80246-74-8
ethyl 3,3,4-trimethylpentanoate

| Conditions | Yield |
|---|---|
| perbromohomoscorpionate copper(I) catalyst In dichloromethane for 3h; | 56% |

| Conditions | Yield |
|---|---|
| With adamantane; indium (III) iodide at 180℃; for 0.5h; Product distribution / selectivity; | 56% |
| indium (III) iodide at 180℃; for 0.5h; Product distribution / selectivity; | 31% |
| zinc(II) iodide at 200℃; for 2h; Product distribution / selectivity; | 17% |
| With indium (III) iodide at 180℃; for 0.5h; | |
| indium (III) iodide at 150 - 200℃; for 0.25 - 2h; Product distribution / selectivity; | 0.5% |
-
-
957188-75-9
[N-(trifluoromethylsulfonyl)imino][4-(trifluoromethyl)phenyl]-λ3-bromane

-
-
79-29-8
2,3-dimethylbutane

-
-
1361955-88-5
C7H14F3NO2S

| Conditions | Yield |
|---|---|
| With 1,1,1,3',3',3'-hexafluoro-propanol at 20℃; Inert atmosphere; Neat (no solvent); regioselective reaction; | 55% |

| Conditions | Yield |
|---|---|
| With di-tert-butyl peroxide at 120℃; for 24h; Sealed tube; | 53% |
-
-
79-29-8
2,3-dimethylbutane

-
-
10349-38-9
p-methoxyphenylisocyanide

-
-
58265-35-3
2,2,3-trimethyl-butyric acid anilide

| Conditions | Yield |
|---|---|
| With ferrocene; di-tert-butyl peroxide In 1,2-dichloro-ethane at 80℃; for 12h; Sealed tube; Green chemistry; | 52% |
-
-
79-29-8
2,3-dimethylbutane

-
A
-
354-96-1
perfluoro(2,3-dimethylbutane)

-
B
-
355-04-4
perfluoroisohexane

-
C
-
865-71-4
perfluoro(3-methylpentane)

-
D
-
1805-22-7
perfluoro(2-methylcyclopentane)

-
E
-
112156-74-8
perfluoro(2,2-dimethylbutane)

| Conditions | Yield |
|---|---|
| cobalt (III) fluoride at 360℃; for 3h; Product distribution; | A 5% B 21% C 7% D 17% E 50% |

-
-
79-29-8
2,3-dimethylbutane

-
A
-
354-96-1
perfluoro(2,3-dimethylbutane)

-
B
-
355-04-4
perfluoroisohexane

-
C
-
1805-22-7
perfluoro(2-methylcyclopentane)

-
D
-
112156-74-8
perfluoro(2,2-dimethylbutane)

| Conditions | Yield |
|---|---|
| cobalt (III) fluoride at 360℃; for 3h; Further byproducts given; | A 5% B 21% C 17% D 50% |

-
-
79-29-8
2,3-dimethylbutane

-
A
-
355-04-4
perfluoroisohexane

-
B
-
865-71-4
perfluoro(3-methylpentane)

-
C
-
1805-22-7
perfluoro(2-methylcyclopentane)

-
D
-
112156-74-8
perfluoro(2,2-dimethylbutane)

| Conditions | Yield |
|---|---|
| cobalt (III) fluoride at 360℃; for 3h; Further byproducts given; | A 21% B 7% C 17% D 50% |

-
-
79-29-8
2,3-dimethylbutane

-
-
119353-21-8
1-octynyl trifluoromethyl sulfone

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile) for 24h; Heating; | 49% |
2,3-Dimethylbutane Chemical Properties
Molecular Structure of 2,3-Dimethylbutane (CAS NO.79-29-8):
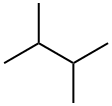
EINECS: 201-193-6
IUPAC Name: 2,3-Dimethylbutane
Molecular Formula: C6H14
Molecular Weight: 86.175360 g/mol
XLogP3: 3.4
H-Bond Donor: 0
H-Bond Acceptor: 0
Canonical SMILES: CC(C)C(C)C
InChI: InChI=1S/C6H14/c1-5(2)6(3)4/h5-6H,1-4H3
InChIKey: ZFFMLCVRJBZUDZ-UHFFFAOYSA-N
Index of Refraction: 1.38
Molar Refractivity: 29.76 cm3
Molar Volume: 128.3 cm3
Surface Tension: 18.3 dyne/cm
Density: 0.671 g/cm3
Enthalpy of Vaporization: 27.38 kJ/mol
Boiling Point: 58.7 °C at 760 mmHg
Vapour Pressure: 220 mmHg at 25 °C
Melting Point: -129 °C
Water Solubility: 44.29 mg/L at 25 °C
Refractive Index: n20/D 1.375(lit.)
Storage Temp.: Flammables area
BRN: 1730737
2,3-Dimethylbutane Consensus Reports
Reported in EPA TSCA Inventory.
2,3-Dimethylbutane Safety Profile
Safety Information of 2,3-Dimethylbutane (CAS NO.79-29-8):
Hazard Codes: F  ,Xn
,Xn 
Risk Statements: 11-65
R11:Highly flammable
R65:Harmful: may cause lung damage if swallowed
Safety Statements: 16-23-33-62
S16:Keep away from sources of ignition
S23:Do not breathe vapour
S33:Take precautionary measures against static discharges
S62:If swallowed, do not induce vomitting; seek medical advice immediately and show this container or label
RIDADR: UN 2457 3/PG 2
WGK Germany: 3
RTECS: EJ9350000
HazardClass: 3
PackingGroup: II
Probably an irritant and narcotic in high concentration. A very dangerous fire and explosion hazard when exposed to heat or flame; can react vigorously with oxidizing materials. Keep away from heat and open flame. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes.
2,3-Dimethylbutane Standards and Recommendations
OSHA PEL: TWA 500 ppm; STEL 1000 ppm
ACGIH TLV: TWA 500 ppm; STEL 1000 ppm
DFG MAK: 200 ppm (720 mg/m3)
NIOSH REL: TWA (Alkanes) 350 mg/m3
DOT Classification: 3; Label: Flammable Liquid
2,3-Dimethylbutane Specification
2,3-Dimethylbutane is a colourless liquid with CAS Registry Number of 79-29-8. It is an isomer of hexane, also known as 1,1,2,2-Tetramethylethane ; Biisopropyl ; Butane, 2,3-dimethyl- ; CCRIS 6020 ; Diisopropyl ; HSDB 76 ; NSC 24837 ; 2,3-Dimethylbutane [UN2457] [Flammable liquid] ; UN2457 . It is incompatible with strong oxidizing agents. When heated to decomposition, it yields Carbon monoxide , Carbon dioxide . 2,3-Dimethylbutane is used as gas chromatography standard.
Related Products
- 20,22-DIHYDRODIGITOXIN
- 20,29,30-Trinorlupane,(17alpha)-
- 20-ETHYL-6-β,8-DIHYDROXY-1-α-METHOXY-4-METHYLHETERATISAN-14-ONE
- 20-Ethylprostaglandin F2-alpha
- 20-Isopropylcholanthrene
- 20-METHYLCHOLANTHREN-15-ONE
- 20-METHYLCHOLANTHRENE PICRATE
- 20-METHYLCHOLANTHRENE-TRINITRO-BENZENE
- 20(S)-Ginsenoside C-K
- 2,10-DIFLUOROBENZO(rst)PENTAPHENE
- 79-30-1
- 793-06-6
- 79307-93-0
- 79-31-2
- 79313-77-2
- 793-19-1
- 79319-85-0
- 79322-83-1
- 79322-97-7
- 79324-50-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View