-
Name
2,4-Dihydroxyacetophenone
- EINECS 201-945-3
- CAS No. 89-84-9
- Article Data161
- CAS DataBase
- Density 1.291 g/cm3
- Solubility
- Melting Point 143-144.5 °C(lit.)
- Formula C8H8O3
- Boiling Point 319.3 °C at 760 mmHg
- Molecular Weight 152.15
- Flash Point 161.1 °C
- Transport Information
- Appearance yellow-brown to reddish-brown fine cryst. powder
- Safety 26-36-24/25
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 4-Acetylresorcinol;Resacetophenone;2,4-Dihydroxyacetophenone;
- PSA 57.53000
- LogP 1.30040
Synthetic route
-
-
65490-08-6
2-hydroxy-4-(methoxymethoxy)acetophenone

-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

| Conditions | Yield |
|---|---|
| H6P2W18O62; silica gel In tetrahydrofuran; methanol at 70℃; for 0.75h; Product distribution; Further Variations:; Catalysts; Solvents; Temperatures; | 100% |
| With toluene-4-sulfonic acid In neat (no solvent, solid phase) at 20℃; for 0.583333h; Green chemistry; | 90% |

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate for 0.0333333h; microwave irradiation; | 98% |
| With iron(III) chloride for 0.0221667h; Friedel Crafts acylation; Microwave irradiation; regioselective reaction; | 98% |
| With zinc(II) chloride Reflux; | 98% |
-
-
72712-19-7
2,4-diacetoxy acetophenone

-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

| Conditions | Yield |
|---|---|
| With water; sodium acetate In ethanol for 5h; Reflux; | 98% |

| Conditions | Yield |
|---|---|
| With acyltransferase from bacterium Pseudomonas sp.YGJ3 In aq. phosphate buffer; dimethyl sulfoxide at 35℃; for 18h; pH=7.5; Friedel-Crafts Acylation; Enzymatic reaction; regioselective reaction; | 97% |

-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In neat (no solvent, solid phase) at 20℃; for 0.583333h; Green chemistry; | 95% |

| Conditions | Yield |
|---|---|
| With periodic acid at 20℃; for 0.0833333h; | 92% |

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate; acetic acid Inert atmosphere; | 91% |

| Conditions | Yield |
|---|---|
| With acetic acid; zinc(II) chloride at 60℃; for 4h; Temperature; Large scale; | 90.6% |
| With PPA Reflux; | 68% |
| With phosphorus pentoxide; silica gel at 100℃; for 24h; | 65% |
-
-
29682-12-0
1-[4-(benzyloxy)-2-hydroxyphenyl]ethanone

-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

| Conditions | Yield |
|---|---|
| With formic acid; sodium formate; palladium on activated charcoal In methanol for 1h; Product distribution; Heating; other aryl benzyl ethers; | 90% |
| With formic acid; sodium formate; palladium on activated charcoal In methanol for 1h; Heating; | 90% |
-
-
108-24-7
acetic anhydride

-
-
108-46-3
recorcinol

-
A
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
B
-
2163-12-4
2,4-diacetylresorcinol

-
C
-
2161-85-5
1-(5-acetyl-2,4-dihydroxyphenyl)-1-ethanone

| Conditions | Yield |
|---|---|
| With zinc(II) chloride at 145 - 150℃; for 0.25h; | A 2% B 7% C 90% |


| Conditions | Yield |
|---|---|
| With pyridinium p-toluenesulfonate for 0.0333333h; microwave irradiation; | 80% |
-
-
40815-74-5
4-allyloxy-2-hydroxyacetophenone

-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

| Conditions | Yield |
|---|---|
| With iodine In dimethyl sulfoxide at 130℃; for 0.5h; | 79% |

| Conditions | Yield |
|---|---|
| With zinc at 64 - 66℃; for 0.0208333h; Friedel-Crafts acylation; microwave irradiation; | 77% |

| Conditions | Yield |
|---|---|
| With acetic acid; zinc(II) chloride at 20℃; for 12h; | 73% |
| With sulfuric acid; acetic acid In di-isopropyl ether; water | 72% |
| With sulfuric acid; acetic anhydride; acetic acid In water | 68% |

| Conditions | Yield |
|---|---|
| With 1H-imidazole; acyltransferase from bacterium Pseudomonas sp.YGJ3 In aq. phosphate buffer at 35℃; for 24h; pH=8.3; Friedel-Crafts Acylation; Enzymatic reaction; regioselective reaction; | 68% |
| With 1H-imidazole; acyltransferase from pseudomonas protegens In aq. buffer at 35℃; for 18h; pH=8.3; Catalytic behavior; Reagent/catalyst; Concentration; pH-value; Friedel-Crafts Acylation; | 98 %Chromat. |
| With 1H-imidazole; acyltransferase from Pseudomonas protegens W211A mutant In aq. phosphate buffer; acetonitrile at 35℃; for 1.5h; pH=7.5; Catalytic behavior; Reagent/catalyst; Friedel-Crafts Acylation; Enzymatic reaction; | 66 %Chromat. |
-
-
2161-86-6
2,4-diacetylphloroglucinol

-
-
108-46-3
recorcinol

-
A
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
B
-
108-73-6
3,5-dihydroxyphenol

| Conditions | Yield |
|---|---|
| With cell-free E. coli extract containing the recombinant acyltransferase from Pseudomonas protegens In aq. phosphate buffer; dimethyl sulfoxide at 35℃; for 0.5h; pH=7.5; Catalytic behavior; Solvent; pH-value; Temperature; Enzymatic reaction; | A 65% B n/a |

-
-
1888-33-1
N-acetyl-(4-methylbenzene)sulfonamide

-
-
108-46-3
recorcinol

-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

| Conditions | Yield |
|---|---|
| With zinc(II) chloride at 110 - 120℃; | 60% |

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In benzene for 3h; crossover Friess rearrangement; Heating; | 60% |
| With 1H-imidazole; acyltransferase from pseudomonas protegens In aq. buffer at 35℃; for 18h; pH=8.3; Friedel-Crafts Acylation; | > 99 %Chromat. |
-
-
72712-19-7
2,4-diacetoxy acetophenone

-
A
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
B
-
52751-42-5
2'-acetoxy-4'-hydroxyacetophenone

| Conditions | Yield |
|---|---|
| With Candida cylindracea lipase In di-isopropyl ether; butan-1-ol at 28 - 30℃; for 11h; | A 40% B 50% |

| Conditions | Yield |
|---|---|
| With acyltransferase from bacterium Pseudomonas sp.YGJ3 In aq. phosphate buffer at 35℃; for 18h; pH=7.5; Friedel-Crafts Acylation; Enzymatic reaction; regioselective reaction; | 41% |
-
-
829-20-9
2',4'-dimethoxyacetophenone

-
A
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
B
-
552-41-0
1-(2-hydroxy-4-methoxyphenyl)ethanone

-
C
-
493-33-4
1-(4-hydroxy-2-methoxy-phenyl)ethanone

| Conditions | Yield |
|---|---|
| With 1-methyl-pyrrolidin-2-one; potassium carbonate; thiophenol for 0.5h; Heating; | A 22% B 16.3% C 38% |

-
-
961-29-5, 13745-20-5
isoliquirtigenin

-
A
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
B
-
64687-96-3
5-hydroxy-2-(4-hydroxphenyl)chroman-4-one

-
C
-
123-08-0
4-hydroxy-benzaldehyde

-
D
-
85359-61-1
chalaurenol

| Conditions | Yield |
|---|---|
| With Tris-HCl buffer; peroxidase from Amorpha fruticosa In 2-methoxy-ethanol at 30℃; for 3h; | A n/a B n/a C n/a D 25% |
| With Tris-HCl buffer; peroxidase from Amorpha fruticosa In 2-methoxy-ethanol at 30℃; for 3h; Mechanism; | A n/a B n/a C n/a D 25% |

-
-
480-66-0
2,4,6-trihydroxyacetophenone

-
-
108-46-3
recorcinol

-
A
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
B
-
108-73-6
3,5-dihydroxyphenol

| Conditions | Yield |
|---|---|
| With cell-free E. coli extract containing the recombinant acyltransferase from Pseudomonas protegens In aq. phosphate buffer; dimethyl sulfoxide at 35℃; for 0.5h; pH=7.5; Enzymatic reaction; | A 13% B n/a |

-
-
90-33-5, 79566-13-5
7-hydroxy-4-methyl-chromen-2-one

-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

| Conditions | Yield |
|---|---|
| With potassium carbonate |

| Conditions | Yield |
|---|---|
| With aluminium trichloride at 185℃; | |
| With zinc(II) chloride |

| Conditions | Yield |
|---|---|
| With aluminium trichloride at 185℃; |

-
A
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
B
-
64-19-7
acetic acid

-
C
-
829-20-9
2',4'-dimethoxyacetophenone

-
D
-
108-46-3
recorcinol

-
-
643-57-2
4,7-dihydroxy-3-[3,7,11-trimethyl-2(E),6(E),10-dodecatrienyl]coumarin

-
A
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
B
-
23687-21-0
4,7-dihydroxy-3-methyl-2H-chromen-2-one

| Conditions | Yield |
|---|---|
| at 230℃; under 0.3 Torr; |
-
-
110-87-2
3,4-dihydro-2H-pyran

-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
-
111841-07-7
2-hydroxy-4-(tetrahydropyran-2-yloxy)acetophenone

| Conditions | Yield |
|---|---|
| With pyridinium p-toluenesulfonate In dichloromethane for 1.5h; Ambient temperature; | 100% |
| With pyridinium p-toluenesulfonate In dichloromethane at 20℃; for 4h; | 98.3% |
| With pyridinium p-toluenesulfonate In dichloromethane for 12h; Ambient temperature; | 90% |
-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
-
100-39-0
benzyl bromide

-
-
22877-01-6
2,4-bis(benzyloxy)acetophenone

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile for 48h; Heating / reflux; | 100% |
| Stage #1: 2',4'-dihydroxy-4-acetophenone With potassium carbonate In acetonitrile at 85℃; for 0.0833333h; Inert atmosphere; Stage #2: benzyl bromide at 85℃; for 12h; Inert atmosphere; | 99% |
| With potassium carbonate In acetonitrile for 18h; Heating; | 97% |

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 0 - 20℃; for 3h; | 100% |
| With triethylamine In tetrahydrofuran at 0 - 20℃; for 3h; | 100% |
| With triethylamine In tetrahydrofuran for 3h; Ambient temperature; | 94% |
| With triethylamine In tetrahydrofuran at 0 - 20℃; for 3h; | 105 g |
| With triethylamine In tetrahydrofuran at 0 - 20℃; for 3h; |
-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
-
100-39-0
benzyl bromide

-
-
29682-12-0
1-[4-(benzyloxy)-2-hydroxyphenyl]ethanone

| Conditions | Yield |
|---|---|
| Stage #1: 2',4'-dihydroxy-4-acetophenone With potassium carbonate In acetonitrile for 1h; Reflux; Stage #2: benzyl bromide In acetonitrile Reflux; | 99% |
| Stage #1: 2',4'-dihydroxy-4-acetophenone With potassium carbonate; potassium iodide In acetone at 50℃; for 0.5h; Stage #2: benzyl bromide In acetone for 5h; Reflux; | 97% |
| With potassium carbonate In acetone for 24h; Reflux; | 93% |
-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
-
107-30-2
chloromethyl methyl ether

-
-
65490-08-6
2-hydroxy-4-(methoxymethoxy)acetophenone

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane for 4.5h; | 99% |
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; | 96.8% |
| With potassium carbonate In acetone for 0.5h; | 95.6% |
-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
-
1228569-46-7
1-(2,4-dihydroxyphenyl)ethylamine

| Conditions | Yield |
|---|---|
| With formate dehydrogenase; Arthrobacter citreus S9 ω-transaminase; ATA-113 ω-transaminase; sodium formate; isopropylamine; NADH; yeast alcohol dehydrogenase pH=7; aq. phosphate buffer; Enzymatic reaction; optical yield given as %ee; | 99% |
-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
-
18162-48-6
tert-butyldimethylsilyl chloride

| Conditions | Yield |
|---|---|
| With 1H-imidazole; dmap In dichloromethane at 0℃; Inert atmosphere; | 99% |
| With 1H-imidazole In N,N-dimethyl-formamide at 40℃; for 5.5h; | 78% |
| With 1H-imidazole In N,N-dimethyl-formamide at 40℃; for 5.5h; |
-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
-
13057-17-5
bromethyl methyl ether

-
-
65490-08-6
2-hydroxy-4-(methoxymethoxy)acetophenone

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; for 12h; Inert atmosphere; | 99% |
| With potassium carbonate In acetone at 50℃; | 64% |
| With potassium carbonate In acetone Reflux; | 59% |
-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
-
98-88-4
benzoyl chloride

-
-
66832-97-1
2,4-dibenzoyloxyacetophenone

| Conditions | Yield |
|---|---|
| With pyridine In diethyl ether at 0℃; for 1h; | 98.5% |
| With pyridine at 20℃; for 2h; Acylation; | 98% |
| With pyridine for 1h; | 83% |
-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
-
107-30-2
chloromethyl methyl ether

-
-
6515-05-5
2',4'-bis(methoxymethoxy)acetophenone

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In N-methyl-acetamide; (2S)-N-methyl-1-phenylpropan-2-amine hydrate | 98.3% |
| With N-ethyl-N,N-diisopropylamine In acetonitrile at 20℃; for 6.5h; | 98% |
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide for 5h; Ambient temperature; | 90% |
-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
-
71243-12-4
1-(2,4-dihydroxy-3-iodophenyl)ethanone

| Conditions | Yield |
|---|---|
| With potassium iodate; iodine In ethanol; water at 20℃; Inert atmosphere; | 98% |
| With potassium iodate; iodine In ethanol; water at 20℃; Inert atmosphere; regiospecific reaction; | 98% |
| With potassium iodate; iodine In ethanol; water at 20℃; Inert atmosphere; regioselective reaction; | 98% |

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride; sodium acetate In water at 100℃; for 6h; | 98% |
| With hydroxylamine hydrochloride; sodium acetate In water for 6h; Reflux; | 98% |
| With hydroxylamine hydrochloride; sodium acetate In water at 70℃; for 6h; | 79% |

| Conditions | Yield |
|---|---|
| With zirconyl nitrate In acetone at 50℃; for 5h; | 98% |
| With yttrium(lll) nitrate hexahydrate In acetic acid at 20℃; for 3h; | 94% |
| With nitric acid; acetic acid at 20℃; for 7h; regioselective reaction; | 53.7% |
-
-
37859-24-8
2-(4-bromophenyl)acetyl chloride

-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
-
112030-39-4
3-(p-bromophenyl)-7-(p-bromophenylacetoxy)-4-methylcoumarin

| Conditions | Yield |
|---|---|
| With tetra(n-butyl)ammonium hydrogensulfate; potassium carbonate In benzene for 4h; Ambient temperature; | 98% |
-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
-
76513-69-4
(2-trimethylethylsilylethoxy)methyl chloride

-
-
107913-74-6
2,4-bis<<2-(trimethylsilyl)ethoxy>methoxy>acetophenone

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In benzene at 110℃; for 3h; | 98% |
-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
-
106-95-6
allyl bromide

-
-
2079-52-9
1-(2,4-bis(allyloxy)phenyl)ethanone

| Conditions | Yield |
|---|---|
| With potassium carbonate Inert atmosphere; | 98% |
| With potassium carbonate In N,N-dimethyl-formamide | 98% |
| With potassium carbonate; sodium iodide In N,N-dimethyl-formamide at 60℃; for 5h; Inert atmosphere; | 98% |
| With caesium carbonate In N,N-dimethyl-formamide at 60℃; for 5h; | 98% |
| With potassium carbonate In acetone for 12h; Heating; | 59.2% |
-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
-
103-80-0
phenylacetyl chloride

-
-
112030-33-8
4-methyl-3-phenyl-7-phenylacetoxy coumarin

| Conditions | Yield |
|---|---|
| With tetra(n-butyl)ammonium hydrogensulfate; potassium carbonate In benzene for 4h; Ambient temperature; | 98% |
-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
-
67-64-1
acetone

-
-
17771-33-4
7-hydroxy-2,2-dimethyl-chroman-4-one

| Conditions | Yield |
|---|---|
| With piperidine for 0.166667h; Neat (no solvent); Microwave irradiation; | 98% |
| With Amberlite IRA 400 basic resin for 0.0833333h; Microwave irradiation; Neat (no solvent); | 89% |
| With pyrrolidine In toluene for 14h; Heating; | 86.7% |
-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
-
5470-11-1
hydroxylamine hydrochloride

-
-
111364-29-5
(1Z)-1-(2,4-dihydroxyphenyl)ethanone oxime

| Conditions | Yield |
|---|---|
| In pyridine at 20℃; | 98% |
-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
-
78-93-3
butanone

-
-
59258-08-1
2-ethyl-7-hydroxy-2-methyl-chroman-4-one

| Conditions | Yield |
|---|---|
| With piperidine for 0.166667h; Neat (no solvent); Microwave irradiation; | 98% |
| With Amberlite IRA 400 basic resin for 0.133333h; Microwave irradiation; Neat (no solvent); | 85% |
-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
-
106-96-7
propargyl bromide

-
-
22287-70-3
1-(2,4-bis(prop-2-yn-1-yloxy)phenyl)ethan-1-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 12h; Reflux; | 98% |
| With potassium carbonate In acetone for 48h; Reflux; | |
| With potassium carbonate In toluene for 12h; Reflux; | |
| Stage #1: 2',4'-dihydroxy-4-acetophenone With potassium carbonate In N,N-dimethyl-formamide at 0℃; for 0.25h; Inert atmosphere; Stage #2: propargyl bromide In N,N-dimethyl-formamide; toluene at 0 - 20℃; for 17h; Inert atmosphere; |
-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
-
19363-41-8
(E)-4-(1-hydrazonoethyl)benzene-1,3-diol

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In N,N-dimethyl-formamide at 20℃; for 4h; | 97% |
| With hydrazine hydrate; acetic acid In methanol at 20℃; | 82% |
| With ethanol; hydrazine | |
| With hydrazine hydrate In water for 0.0333333h; Microwave irradiation; |
-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
-
108-24-7
acetic anhydride

-
-
72712-19-7
2,4-diacetoxy acetophenone

| Conditions | Yield |
|---|---|
| With magnesium(II) perchlorate at 20℃; for 0.5h; | 97% |
| With pyridine; aluminum oxide at 120 - 122℃; for 1h; microwave irradiation; | 54% |
| With sodium acetate bei 0.5 Minuten langem Kochen; |
-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
-
870-63-3
prenyl bromide

-
-
24672-83-1
1-[2-hydroxy-4-(3-methylbut-2-enyloxy)phenyl]ethanone

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 80℃; Williamson Ether Synthesis; | 97% |
| With potassium carbonate In acetone for 8h; Reflux; | 89% |
| With potassium carbonate In acetone at 80℃; for 6h; | 82% |
-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
-
75-03-6
ethyl iodide

-
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 80℃; for 6h; | 97% |
| With potassium carbonate In N,N-dimethyl-formamide for 4h; | 63% |
-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
-
79-30-1
isobutyryl chloride

-
-
957272-64-9
2',4'-di-(2-methylpropanoyloxy)-acetophenone

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; | 97% |
-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
-
108-94-1
cyclohexanone

-
-
62756-43-8
7-hydroxy-spiro<2H-1-benzopyran-2,1'-cyclohexane>-4(3H)-one

| Conditions | Yield |
|---|---|
| With piperidine for 0.166667h; Neat (no solvent); Microwave irradiation; | 97% |
| With Amberlite IRA 400 basic resin for 0.1h; Microwave irradiation; Neat (no solvent); | 90% |
| With pyrrolidine In acetonitrile at 45℃; for 8h; | 84% |
2',4'-Dihydroxyacetophenone Chemical Properties
Molecule structure of 2,4-Dihydroxyacetophenone (CAS NO.89-84-9):
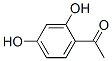
IUPAC Name: 1-(2,4-Dihydroxyphenyl)ethanone
Molecular Weight: 152.14732 g/mol
Molecular Formula: C8H8O3
Density: 1.18 g/mL at 25 °C(lit.)
Melting Point: 143-144.5 °C(lit.)
Boiling Point: 319.3 °C at 760 mmHg
Flash Point: 161.1 °C
Index of Refraction: 1.595
Molar Refractivity: 40.04 cm3
Molar Volume: 117.8 cm3
Polarizability: 15.87×10-24 cm3
Surface Tension: 56.1 dyne/cm
Enthalpy of Vaporization: 58.32 kJ/mol
Vapour Pressure: 0.000183 mmHg at 25 °C
Sensitive Moisture: sensitive
XLogP3: 1.7
H-Bond Donor: 2
H-Bond Acceptor: 3
Rotatable Bond Count: 1
Tautomer Count: 21
Exact Mass: 152.047344
MonoIsotopic Mass: 152.047344
Topological Polar Surface Area: 57.5
Heavy Atom Count: 11
Complexity: 155
Canonical SMILES: CC(=O)C1=C(C=C(C=C1)O)O
InChI: InChI=1S/C8H8O3/c1-5(9)7-3-2-6(10)4-8(7)11/h2-4,10-11H,1H3
InChIKey: SULYEHHGGXARJS-UHFFFAOYSA-N
EINECS: 201-945-3
Product Categories: Acetophenone Series; Aromatic Acetophenones & Derivatives (substituted); Benzene series
2',4'-Dihydroxyacetophenone Uses
2,4-Dihydroxyacetophenone (CAS NO.89-84-9) is used as intermediates in organic synthesis.
2',4'-Dihydroxyacetophenone Production
2,4-Dihydroxyacetophenone is made by acetylation of acetic acid and resorcinol .
2',4'-Dihydroxyacetophenone Toxicity Data With Reference
| 1. | eye-rbt 500 mg SEV | IHFCAY Industrial Hygiene Foundation of America, Chemical and Toxicological Series, Bulletin. 6 (1967),1. | ||
| 2. | orl-rat LD50:2830 mg/kg | IHFCAY Industrial Hygiene Foundation of America, Chemical and Toxicological Series, Bulletin. 6 (1967),1. |
2',4'-Dihydroxyacetophenone Consensus Reports
Reported in EPA TSCA Inventory.
2',4'-Dihydroxyacetophenone Safety Profile
Hazard Codes:  Xi
Xi
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36-24/25
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
S24/25:Avoid contact with skin and eyes.
WGK Germany: 2
RTECS: AM7525000
Hazard Note: Irritant
HS Code: 29145000
Moderately toxic by ingestion. Experimental reproductive effects. A severe eye irritant. When heated to decomposition it emits acrid smoke and irritating fumes.
2',4'-Dihydroxyacetophenone Specification
2,4-Dihydroxyacetophenone (CAS NO.89-84-9) is also named as 1-(2,4-Dihydroxyphenyl)ethanone ; 2,4-Dihydroxyacetophenone ; 4-08-00-01792 (Beilstein Handbook Reference) ; 4-Acetylresorcinol ; AI3-00866 ; BRN 1282505 ; NSC 10883 ; Resacetophenone ; Resoacetophenone ; Resorcinol, 4-acetyl- ; UNII-UC3V356VZC . 2,4-Dihydroxyacetophenone (CAS NO.89-84-9) is yellow-brown to reddish-brown fine cryst. It is soluble in hot alcohol, pyridine, and glacial acetic acid, almost insoluble in ether, benzene and chloroform.
Related Products
- 20,22-DIHYDRODIGITOXIN
- 20,29,30-Trinorlupane,(17alpha)-
- 20-ETHYL-6-β,8-DIHYDROXY-1-α-METHOXY-4-METHYLHETERATISAN-14-ONE
- 20-Ethylprostaglandin F2-alpha
- 20-Isopropylcholanthrene
- 20-METHYLCHOLANTHREN-15-ONE
- 20-METHYLCHOLANTHRENE PICRATE
- 20-METHYLCHOLANTHRENE-TRINITRO-BENZENE
- 20(S)-Ginsenoside C-K
- 2,10-DIFLUOROBENZO(rst)PENTAPHENE
- 89850-72-6
- 89850-95-3
- 89851-99-0
- 898532-85-9
- 898537-18-3
- 898543-06-1
- 898546-82-2
- 898566-17-1
- 898598-33-9
- 89-86-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View