-
Name
2,5-Dichloronitrobenzene
- EINECS 201-923-3
- CAS No. 89-61-2
- Article Data51
- CAS DataBase
- Density 1.533 g/cm3
- Solubility Soluble in water, ethanol, ether, benzene, carbon disulfide. Slightly soluble in carbon tetrachloride.
- Melting Point 52-54 °C(lit.)
- Formula C6H3Cl2NO2
- Boiling Point 267 °C at 760 mmHg
- Molecular Weight 192.001
- Flash Point 109.4 °C
- Transport Information UN 3077 9/PG 3
- Appearance yellow flakes
- Safety 26-60
- Risk Codes 22-36-51/53
-
Molecular Structure
-
Hazard Symbols
 Xn;
Xn;  N
N
- Synonyms 2,5-Dichloronitrobenzol;2,5-Dichloro-1-nitrobenzene;Benzene, 2,5-dichloronitro-;Benzene, 1,4-dichloro-2-nitro-;2,5-Dichlornitrobenzen [Czech];2, 5-Dichlornitrobenzen;1-Nitro-2,5-dichlorobenzene;Nitro-p-dichlorobenzene;1,4-dichloro-2-nitro-benzene;1,4-Dichloro-2-nitrobenzene;
- PSA 45.82000
- LogP 3.42480
Synthetic route

| Conditions | Yield |
|---|---|
| With fluoro alcohol In water; ethyl acetate; acetonitrile at -20℃; for 0.0833333h; | 97% |

| Conditions | Yield |
|---|---|
| With ortho-difluorobenzene; sulfuric acid; nitric acid at 35℃; Temperature; | 96.7% |
| With sulfuric acid; nitric acid at 0 - 23℃; for 0.283333h; Inert atmosphere; | 93% |
| With nitric acid |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; oxygen; copper(l) chloride In dimethyl sulfoxide at 160℃; under 760.051 Torr; for 30h; Schlenk technique; Sealed tube; | 78% |
| With 2.9-dimethyl-1,10-phenanthroline; oxygen; copper (I) acetate; silver sulfate; sodium chloride In dimethyl sulfoxide at 160℃; for 24h; Schlenk technique; | 54% |
| With 2.9-dimethyl-1,10-phenanthroline; oxygen; copper diacetate; silver sulfate; sodium chloride In dimethyl sulfoxide at 160℃; under 760.051 Torr; for 20h; Schlenk technique; | 54% |
-
-
27165-22-6
2-nitro-4-chlorobenzenediazonium cation

-
A
-
56978-50-8
4,4'-dichloro-2,2'-dinitrobiphenyl

-
B
-
89-61-2
2,5-dichloronitrobenzene

| Conditions | Yield |
|---|---|
| With hydrogenchloride; copper dichloride |
-
-
10203-04-0
3,6-dichloro-2-nitro-benzaldehyde

-
-
89-61-2
2,5-dichloronitrobenzene

| Conditions | Yield |
|---|---|
| With potassium hydroxide at 100℃; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; acetic acid at 40℃; anschliessendes Behandeln mit Kupfer(I)-chlorid und konz. wss. Salzsaeure bei 70grad; |

| Conditions | Yield |
|---|---|
| With antimonypentachloride beim Chlorieren; |

| Conditions | Yield |
|---|---|
| With antimonypentachloride bei der Chlorierung; |
-
-
121-73-3
3-Chloronitrobenzene

-
A
-
99-54-7
3,4-dichloronitrobenzene

-
B
-
3209-22-1
1,2-Dichloro-3-nitrobenzene

-
C
-
89-61-2
2,5-dichloronitrobenzene

| Conditions | Yield |
|---|---|
| With chlorine; iron(III) chloride at 90℃; Product distribution; relative rates; |

-
-
98-95-3
nitrobenzene

-
A
-
99-54-7
3,4-dichloronitrobenzene

-
B
-
121-73-3
3-Chloronitrobenzene

-
C
-
3209-22-1
1,2-Dichloro-3-nitrobenzene

-
D
-
100-00-5
4-chlorobenzonitrile

-
E
-
88-73-3
2-Chloronitrobenzene

-
F
-
89-61-2
2,5-dichloronitrobenzene

| Conditions | Yield |
|---|---|
| With chlorine; iron(III) chloride at 60 - 120℃; Product distribution; Kinetics; relative rates of each steps; |

-
-
88-73-3
2-Chloronitrobenzene

-
A
-
3209-22-1
1,2-Dichloro-3-nitrobenzene

-
B
-
606-21-3
1-chloro-2,6-dinitrobenzene

-
C
-
97-00-7
1-chloro-2,4-dinitro-benzene

-
D
-
89-61-2
2,5-dichloronitrobenzene

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sulfuric acid; nitric acid at 25℃; Product distribution; | A 0.2 % Chromat. B 6.7 % Chromat. C 94.8 % Chromat. D 0.3 % Chromat. |

-
-
88-73-3
2-Chloronitrobenzene

-
A
-
3209-22-1
1,2-Dichloro-3-nitrobenzene

-
B
-
89-61-2
2,5-dichloronitrobenzene

| Conditions | Yield |
|---|---|
| With chlorine; iron(III) chloride at 90℃; Product distribution; relative rates; |

| Conditions | Yield |
|---|---|
| With antimonypentachloride; Nitryl chloride In dichloromethane at 20℃; for 0.25h; | A 96 % Chromat. B 4 % Chromat. |
-
-
7782-50-5
chlorine

-
-
7705-08-0
iron(III) chloride

-
-
98-95-3
nitrobenzene

-
-
89-61-2
2,5-dichloronitrobenzene

| Conditions | Yield |
|---|---|
| at 20℃; |

-
-
7782-50-5
chlorine

-
-
7705-08-0
iron(III) chloride

-
-
98-95-3
nitrobenzene

-
A
-
121-73-3
3-Chloronitrobenzene

-
B
-
89-61-2
2,5-dichloronitrobenzene

| Conditions | Yield |
|---|---|
| at 40 - 42℃; |

-
-
7782-50-5
chlorine

-
-
7705-08-0
iron(III) chloride

-
-
98-95-3
nitrobenzene

-
A
-
121-73-3
3-Chloronitrobenzene

-
B
-
118-74-1
hexachlorobenzene

-
C
-
89-61-2
2,5-dichloronitrobenzene

-
-
7647-18-9
antimonypentachloride

-
-
88-73-3
2-Chloronitrobenzene

-
A
-
611-06-3
2,4-dichloronitrobenzene

-
B
-
89-61-2
2,5-dichloronitrobenzene

| Conditions | Yield |
|---|---|
| at 100℃; durch Chlorierung; |

-
-
10025-91-9
antimony(III) chloride

-
-
7782-50-5
chlorine

-
-
98-95-3
nitrobenzene

-
A
-
88-73-3
2-Chloronitrobenzene

-
B
-
89-61-2
2,5-dichloronitrobenzene

-
-
10025-91-9
antimony(III) chloride

-
-
7782-50-5
chlorine

-
-
98-95-3
nitrobenzene

-
A
-
3209-22-1
1,2-Dichloro-3-nitrobenzene

-
B
-
88-73-3
2-Chloronitrobenzene

-
C
-
89-61-2
2,5-dichloronitrobenzene


| Conditions | Yield |
|---|---|
| at 0℃; Diazotieren und Behandeln mit Kupfer(I)-chlorid und konz.Salzsaeure; |

-
-
121-73-3
3-Chloronitrobenzene

-
A
-
3209-22-1
1,2-Dichloro-3-nitrobenzene

-
B
-
89-61-2
2,5-dichloronitrobenzene

| Conditions | Yield |
|---|---|
| bei der Chlorierung; |

-
-
10203-04-0
3,6-dichloro-2-nitro-benzaldehyde

-
A
-
64-18-6
formic acid

-
B
-
89-61-2
2,5-dichloronitrobenzene


| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: nitrous acid; alcohol 2: antimony chloride / bei der Chlorierung View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: concentrated sulfuric acid; nitric acid / man erhitzt mit Ammoniak im geschlossenen Rohr auf 160grad und trennt die beiden entstandenen Chlor-nitro-aniline durch Destillation mit Wasserdampf 2: nitrous acid; alcohol 3: antimony chloride / bei der Chlorierung View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: iodine; chlorine 2: bei der Nitrierung View Scheme |
-
-
98-95-3
nitrobenzene

-
A
-
99-54-7
3,4-dichloronitrobenzene

-
B
-
121-73-3
3-Chloronitrobenzene

-
C
-
89-61-2
2,5-dichloronitrobenzene

| Conditions | Yield |
|---|---|
| With trichloroisocyanuric acid at 200℃; for 5h; Inert atmosphere; |

-
-
787633-79-8
(R,S)-1-(4-chloro-2-nitrophenyl)ethanol

-
-
89-61-2
2,5-dichloronitrobenzene

| Conditions | Yield |
|---|---|
| With 1,10-Phenanthroline; oxygen; potassium carbonate; copper dichloride; silver(l) oxide In dimethyl sulfoxide at 140℃; under 3750.38 Torr; for 36h; Autoclave; | 80 %Chromat. |
-
-
23082-51-1
4'-chloro-2'-nitroacetophenone

-
-
89-61-2
2,5-dichloronitrobenzene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium tetrahydroborate / methanol / 0 °C 2: potassium carbonate; copper dichloride; 1,10-Phenanthroline; oxygen; silver(l) oxide / dimethyl sulfoxide / 36 h / 140 °C / 3750.38 Torr / Autoclave View Scheme |
-
-
110-91-8
morpholine

-
-
89-61-2
2,5-dichloronitrobenzene

-
-
65976-60-5
4-(4-chloro-2-nitro-phenyl)morpholine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 50℃; under 4500360 Torr; for 20h; | 100% |
| at 2℃; Heating; | 50% |

| Conditions | Yield |
|---|---|
| With hydrogen; zinc dibromide; palladium on activated charcoal In ethyl acetate under 760 Torr; | 100% |
| 99% | |
| With platinum on activated charcoal; hydrogen at 60℃; for 1h; Temperature; Inert atmosphere; Autoclave; Supercritical conditions; | 99.4% |

| Conditions | Yield |
|---|---|
| With ammonia at 125℃; under 15001.5 Torr; for 1h; Autoclave; | 99.85% |
-
-
106-48-9
4-chloro-phenol

-
-
89-61-2
2,5-dichloronitrobenzene

-
-
135-12-6
4-chloro-1-(4-chlorophenoxy)-2-nitrobenzene

| Conditions | Yield |
|---|---|
| With potassium hydroxide at 135 - 140℃; for 3.5h; Reagent/catalyst; Temperature; Large scale; | 99.53% |
| With potassium hydroxide In water at 170℃; for 2.5h; Inert atmosphere; | 97% |
| Stage #1: 4-chloro-phenol With sodium hydride In N,N-dimethyl-formamide; mineral oil at 20℃; for 0.666667h; Stage #2: 2,5-dichloronitrobenzene In N,N-dimethyl-formamide; mineral oil at 80℃; for 1.25h; | 93% |
| With sodium hydride In N,N-dimethyl-formamide at 20℃; |
-
-
109-89-7
diethylamine

-
-
89-61-2
2,5-dichloronitrobenzene

-
-
86309-90-2
N-(4-Chloro-2-nitrophenyl)diethylamine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 50℃; under 4500360 Torr; for 20h; | 98% |
| With methanol at 150℃; Kinetik der Reaktion bei 85grad und 110grad; |
-
-
123-75-1
pyrrolidine

-
-
89-61-2
2,5-dichloronitrobenzene

-
-
41173-36-8
5-chloro-2-(1-pyrrolidinyl)nitrobenzene

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 50℃; under 4500360 Torr; for 20h; | 97% |
| at 2℃; Heating; |
-
-
105-56-6
ethyl 2-cyanoacetate

-
-
89-61-2
2,5-dichloronitrobenzene

-
-
65547-99-1
ethyl 2-(4-chloro-2-nitrophenyl)-2-cyanoacetate

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 100℃; | 97% |
| Stage #1: ethyl 2-cyanoacetate With sodium hydride; dimethyl sulfoxide In mineral oil for 0.166667h; Stage #2: 2,5-dichloronitrobenzene In mineral oil at 20℃; for 1h; | 71% |
| With sodium ethanolate 1.) EtOH, 2.) EtOH, reflux, 12 h; Multistep reaction; |
-
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
-
89-61-2
2,5-dichloronitrobenzene

-
-
103748-12-5
2-(4chloro-N-methyl-2-nitranilino)ethanol

| Conditions | Yield |
|---|---|
| In pyridine for 5h; Heating; | 97% |
-
-
110-89-4
piperidine

-
-
89-61-2
2,5-dichloronitrobenzene

-
-
33784-44-0
1-(4-chloro-2-nitrophenyl)piperidine

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 20℃; for 18.5h; | 96.4% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In dimethyl sulfoxide | 96% |
| In ethyl acetate; N,N-dimethyl-formamide | 95% |
| With sodium hydroxide; tetrabutylammomium bromide In water for 30h; Heating; | 81% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 70℃; for 2h; | 95% |
| With sodium hydroxide; copper chloride; glycerol | |
| With zinc hydroxide at 150 - 160℃; | |
| With sodium carbonate at 155 - 165℃; |
-
-
120-83-2
2,4-dichlorophenol

-
-
89-61-2
2,5-dichloronitrobenzene

-
-
2392-48-5
2,4,4'-trichloro-2'-nitrodiphenyl ether

| Conditions | Yield |
|---|---|
| Stage #1: 2,4-dichlorophenol With potassium hydroxide In water at 110℃; Stage #2: 2,5-dichloronitrobenzene In water at 150℃; | 95% |
| With potassium hydroxide | |
| With potassium hydroxide In dimethyl sulfoxide at 100℃; | |
| With potassium hydroxide In water at 125 - 130℃; Large scale; | 4642.3 g |
-
-
17999-25-6
methyl thiosalicylate

-
-
89-61-2
2,5-dichloronitrobenzene

| Conditions | Yield |
|---|---|
| In methanol; sodium hydroxide; water | 95% |

-
-
89-61-2
2,5-dichloronitrobenzene

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In ethanol for 14h; Reflux; | 94% |
-
-
108-98-5
thiophenol

-
-
89-61-2
2,5-dichloronitrobenzene

-
-
4548-56-5
4-chloro-2-nitrophenyl phenyl sulfide

| Conditions | Yield |
|---|---|
| With ammonium acetate In ethanol for 48h; Heating; | 92% |
| With sodium hydroxide In ethanol for 1h; Heating; | 90% |
| With caesium carbonate In acetonitrile at 130℃; for 0.133333h; Microwave irradiation; | 66% |
-
-
99-76-3
methyl 4-hydroxylbenzoate

-
-
89-61-2
2,5-dichloronitrobenzene

-
-
943617-66-1
methyl 4-(4-chloro-2-nitrophenoxy)benzoate

| Conditions | Yield |
|---|---|
| With sodium carbonate In ethanol Heating / reflux; | 92% |
-
-
2637-34-5
2-Mercaptopyridine

-
-
89-61-2
2,5-dichloronitrobenzene

-
-
126395-05-9
2-[(4-chloro-2-nitrophenyl)thio]pyridine

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 150℃; for 0.166667h; Microwave irradiation; | 92% |
| With sodium In methanol at 65℃; for 7h; Inert atmosphere; Reflux; regioselective reaction; | 71% |
-
-
91-60-1
2-Naphthalenethiol

-
-
89-61-2
2,5-dichloronitrobenzene

-
-
1100794-53-3
4-chloro-2-nitro-1-(2-naphthylthio)benzene

| Conditions | Yield |
|---|---|
| With sodium In methanol at 65℃; for 3h; Inert atmosphere; Reflux; regioselective reaction; | 92% |
| With sodium hydroxide In ethanol; water |

| Conditions | Yield |
|---|---|
| With potassium carbonate In tetrahydrofuran at 40℃; for 0.5h; Microwave irradiation; | 92% |
| With sodium hydroxide In ethanol; water |
-
-
84345-15-3
1-benzyl-2-mercapto-1H-imidazolo-5-carboxylic acid

-
-
89-61-2
2,5-dichloronitrobenzene

-
-
108403-90-3
3-Benzyl-2-(4-chloro-2-nitro-phenylsulfanyl)-3H-imidazole-4-carboxylic acid

| Conditions | Yield |
|---|---|
| With sodium In ethanol; N,N-dimethyl-formamide for 1.5h; Heating; | 90% |
-
-
14759-09-2
1-(2-(piperidin-4-yl)ethyl)piperidine

-
-
89-61-2
2,5-dichloronitrobenzene

-
-
1314530-83-0
1-(4-chloro-2-nitro-phenyl)-4-(2-piperidin-1-yl-ethyl)-piperidine

| Conditions | Yield |
|---|---|
| In acetonitrile at 175℃; for 0.5h; Microwave irradiation; | 90% |

| Conditions | Yield |
|---|---|
| In ethanol | 89% |
| With ethanol at 160℃; | |
| at 2℃; Heating; |

| Conditions | Yield |
|---|---|
| With potassium fluoride; polydiallyldimethylammonium chloride In dimethyl sulfoxide at 200℃; for 1h; | 88.6% |
| With potassium fluoride; bis(tricyclohexylphosphine)nickel(II) dichloride; tetrabutyl ammonium fluoride In N,N-dimethyl-formamide at 150℃; for 8h; Inert atmosphere; | 77.2% |
| With potassium fluoride; tetraphenylphosphonium bromide at 145 - 155℃; for 6h; | 56% |

| Conditions | Yield |
|---|---|
| With lithium tert-butoxide In ISOPROPYLAMIDE at -5 - 0℃; for 2h; Product distribution / selectivity; | 88% |
| With sodium t-butanolate In ISOPROPYLAMIDE at -5 - 0℃; for 2h; Product distribution / selectivity; | 86% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 150℃; for 0.166667h; Microwave irradiation; | 88% |
-
-
1311484-52-2
1-phenylvinylboronic acid MIDA ester

-
-
89-61-2
2,5-dichloronitrobenzene

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); potassium carbonate In 1,4-dioxane; water at 120℃; for 0.5h; Suzuki-Miyaura Coupling; | 88% |

| Conditions | Yield |
|---|---|
| With zinc(II) oxide at 130℃; for 0.133333h; Ionic liquid; Microwave irradiation; | 87% |
| With silica gel; caesium carbonate In dimethyl sulfoxide for 0.05h; microwave irradiation; | 77% |
| With silica gel; caesium carbonate In dimethyl sulfoxide at 150℃; for 3.33333h; | 68% |
-
-
141-43-5
ethanolamine

-
-
89-61-2
2,5-dichloronitrobenzene

-
-
59320-13-7
2-(4-chloro-2-nitrophenylamino)ethanol

| Conditions | Yield |
|---|---|
| In butan-1-ol Reflux; | 86% |
| With butan-1-ol | |
| With potassium carbonate | |
| With ethanol at 140℃; | |
| at 2℃; Heating; |
2,5-Dichloronitrobenzene Consensus Reports
2,5-Dichloronitrobenzene Specification
The 1,4-Dichloro-2-nitrobenzene, with the CAS registry number 89-61-2 and EINECS registry number 201-923-3, is also called 2,5-Dichloronitrobenzene. It is a kind of yellow flakes, and belongs to the following proudct categories: Intermediates of Dyes and Pigments; Aromatic Halides (substituted); Benzene derivates. And the molecular formula of this chemical is C6H3Cl2NO2. What's more, it is used as a kind of dye intermediate, and it is also used as nitrogen adjuvant.
The physical properties of 1,4-Dichloro-2-nitrobenzene are as following: (1)ACD/LogP: 2.95; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.95; (4)ACD/LogD (pH 7.4): 2.95; (5)#H bond acceptors: 3; (6)#H bond donors: 0; (7)#Freely Rotating Bonds: 1; (8)Polar Surface Area: 45.82 Å2; (9)Index of Refraction: 1.595; (10)Molar Refractivity: 42.58 cm3; (11)Molar Volume: 125.1 cm3; (12)Polarizability: 16.88×10-24cm3; (13)Surface Tension: 50.9 dyne/cm; (14)Density: 1.533 g/cm3; (15)Flash Point: 109.4 °C; (16)Enthalpy of Vaporization: 48.47 kJ/mol; (17)Boiling Point: 267 °C at 760 mmHg; (18)Vapour Pressure: 0.0138 mmHg at 25°C.
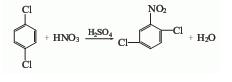
Preparation of 1,4-Dichloro-2-nitrobenzene: It can be prepared by p-dichlorobenzene via nitration, water scrubbing, neutralization and separation.
You should be cautious while dealing with this chemical. It irritates eyes, and it is also harmful if swallowed. What's more, it is very toxic to aquatic organisms, and may cause long-term adverse effects in the aquatic environment. Therefore, you had better take the following instructions: This material and/or its container must be disposed of as hazardous waste, and in case of contacting with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: O=[N+]([O-])c1cc(Cl)ccc1Cl
(2)InChI: InChI=1/C6H3Cl2NO2/c7-4-1-2-5(8)6(3-4)9(10)11/h1-3H
(3)InChIKey: RZKKOBGFCAHLCZ-UHFFFAOYAY
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LD50 | oral | 800mg/kg (800mg/kg) | National Technical Information Service. Vol. OTS0536149, | |
| mouse | LD50 | oral | 2850mg/kg (2850mg/kg) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 25(8), Pg. 50, 1981. | |
| rat | LD50 | oral | 1gm/kg (1000mg/kg) | Zhonghua Yufangyixue Zazhi. Chinese Journal of Preventive Medicine. Vol. 21, Pg. 315, 1987. |
Related Products
- 20,22-DIHYDRODIGITOXIN
- 20,29,30-Trinorlupane,(17alpha)-
- 20-ETHYL-6-β,8-DIHYDROXY-1-α-METHOXY-4-METHYLHETERATISAN-14-ONE
- 20-Ethylprostaglandin F2-alpha
- 20-Isopropylcholanthrene
- 20-METHYLCHOLANTHREN-15-ONE
- 20-METHYLCHOLANTHRENE PICRATE
- 20-METHYLCHOLANTHRENE-TRINITRO-BENZENE
- 20(S)-Ginsenoside C-K
- 2,10-DIFLUOROBENZO(rst)PENTAPHENE
- 896127-80-3
- 89613-32-1
- 89614-96-0
- 89615-42-9
- 89615-73-6
- 896160-35-3
- 89-62-3
- 89624-02-2
- 89630-68-2
- 896-33-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View