-
Name
2-Chloropyrimidine
- EINECS 217-020-2
- CAS No. 1722-12-9
- Article Data42
- CAS DataBase
- Density 1.303 g/cm3
- Solubility Slightly soluble in water. 33.3 mg soluble in 1 mL of alcohol.
- Melting Point 63-66 °C(lit.)
- Formula C4H3ClN2
- Boiling Point 199.2 °C at 760 mmHg
- Molecular Weight 114.534
- Flash Point 109.2 °C
- Transport Information
- Appearance yellowish to pale orange crystals or cryst. powder
- Safety 26-37/39-36
- Risk Codes 22-36-36/38-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, Xi
Xi
- Synonyms 2 Chloro Pyridine;2-Chlorpyrimidin;2-Chloro-4-deoxyuracil;2-Chloropyrimidine D001;Pyrimidine, 2-chloro-;
- PSA 25.78000
- LogP 1.13000
Synthetic route

| Conditions | Yield |
|---|---|
| With trichlorophosphate Chlorination; | 70% |
-
-
131242-36-9
2-sulfanylpyrimidine

-
-
1722-12-9
2-chloropyrimidine

| Conditions | Yield |
|---|---|
| Stage #1: 2-sulfanylpyrimidine With trichloroisocyanuric acid; water; benzyltrimethylammonium chloride In acetonitrile at -30 - -25℃; for 0.5h; Stage #2: With isobutylamine In acetonitrile Further stages.; | 70% |
| With hydrogenchloride; sodium hypochlorite In dichloromethane; water | |
| With thionyl chloride; dihydrogen peroxide In water; acetonitrile at 25℃; |
-
-
1003845-06-4
(2-chloropyrimidin-5-yl)boronic acid

-
-
1722-12-9
2-chloropyrimidine

| Conditions | Yield |
|---|---|
| With 2-bromo-pyridine; tetrakis(triphenylphosphine) palladium(0); sodium carbonate In ethanol; toluene Reflux; | 65% |

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran for 24h; Heating; | A 30% B 50% |

-
-
97-93-8
triethylaluminum

-
-
32779-36-5
5-Bromo-2-chloropyrimidine

-
A
-
1722-12-9
2-chloropyrimidine

-
B
-
111196-81-7
2-chloro-5-ethylpyrimidine

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran for 24h; Heating; | A 30% B 42% |

-
-
3934-20-1
2,6-Dichloropyrimidine

-
-
97-93-8
triethylaluminum

-
A
-
1722-12-9
2-chloropyrimidine

-
B
-
188707-99-5
2-chloro-4-ethylpyrimidine

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran for 24h; Heating; | A 25% B 37% |


| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium nitrite In water at -15 - 0℃; for 2.25h; | 34% |
| With hydrogenchloride; sodium nitrite | |
| With hydrogenchloride; zinc(II) chloride; sodium nitrite In dichloromethane; water | |
| With hydrogenchloride; zinc(II) chloride; sodium nitrite In dichloromethane; water | |
| With hydrogenchloride; copper dichloride; sodium nitrite In dichloromethane |

| Conditions | Yield |
|---|---|
| With zinc | |
| With potassium acetate; palladium diacetate; bis(pinacol)diborane; tricyclohexylphosphine In 1,4-dioxane at 110℃; for 0.166667h; Inert atmosphere; | |
| With hydrogen In methanol; ethanol at 20℃; |
-
-
38353-09-2
2-pyrimidinone hydrochloride

-
-
1722-12-9
2-chloropyrimidine

| Conditions | Yield |
|---|---|
| With phosphorus pentachloride; trichlorophosphate | |
| With trichlorophosphate |
-
-
930-36-9
1-methyl-1H-pyrazole

-
-
67-66-3
chloroform

-
A
-
4513-94-4
1H-pyrrole-2-carbonitrile

-
B
-
1722-12-9
2-chloropyrimidine

-
C
-
51269-82-0
5-chloronicotinonitrile

| Conditions | Yield |
|---|---|
| at 550 - 555℃; Yield given. Title compound not separated from byproducts; | |
| at 550 - 555℃; Title compound not separated from byproducts; |

-
-
10199-67-4
1-benzylpyrazole

-
-
67-66-3
chloroform

-
A
-
1722-12-9
2-chloropyrimidine

-
B
-
7431-45-0
2-phenylpyrimidine

-
C
-
244-76-8
α-carboline

| Conditions | Yield |
|---|---|
| at 550 - 555℃; Title compound not separated from byproducts; |


| Conditions | Yield |
|---|---|
| at 550 - 555℃; Title compound not separated from byproducts; |


| Conditions | Yield |
|---|---|
| at 550 - 555℃; Title compound not separated from byproducts; |

-
-
67-66-3
chloroform

-
-
202344-41-0
1-(pyridin-4-ylmethyl)-1H-pyrazol-5-amine

-
A
-
288-13-1
NH-pyrazole

-
B
-
1722-12-9
2-chloropyrimidine

| Conditions | Yield |
|---|---|
| at 550 - 555℃; Title compound not separated from byproducts; | |
| at 550 - 555℃; |

-
-
67-66-3
chloroform

-
-
202344-37-4
1-(4-chlorobenzyl)pyrazole

-
A
-
288-13-1
NH-pyrazole

-
B
-
1722-12-9
2-chloropyrimidine

-
D
-
191212-20-1
2-(4-chlorophenyl)pyrimidine

| Conditions | Yield |
|---|---|
| at 550 - 555℃; Title compound not separated from byproducts; |

-
-
202344-41-0
1-(pyridin-4-ylmethyl)-1H-pyrazol-5-amine

-
A
-
288-13-1
NH-pyrazole

-
B
-
1722-12-9
2-chloropyrimidine

| Conditions | Yield |
|---|---|
| With chloroform at 550 - 555℃; Title compound not separated from byproducts; |

-
-
202344-37-4
1-(4-chlorobenzyl)pyrazole

-
A
-
288-13-1
NH-pyrazole

-
B
-
1722-12-9
2-chloropyrimidine

-
D
-
191212-20-1
2-(4-chlorophenyl)pyrimidine

| Conditions | Yield |
|---|---|
| With chloroform at 550 - 555℃; Title compound not separated from byproducts; |



| Conditions | Yield |
|---|---|
| With potassium carbonate In ethanol |
-
-
56-05-3
2-Amino-4,6-dichloropyrimidine

-
-
702-23-8
2-(4-Methoxyphenyl)ethanol

-
-
1722-12-9
2-chloropyrimidine

| Conditions | Yield |
|---|---|
| With NaH In tetrahydrofuran |
-
-
56-05-3
2-Amino-4,6-dichloropyrimidine

-
-
18217-00-0
1-(2-Chloroethyl)-4-methoxybenzene

-
-
1722-12-9
2-chloropyrimidine

| Conditions | Yield |
|---|---|
| With Na2S In water; N,N-dimethyl-formamide |

| Conditions | Yield |
|---|---|
| With sodium bicarbonate; trifluoroacetic acid In dichloromethane |

-
-
1722-12-9
2-chloropyrimidine

| Conditions | Yield |
|---|---|
| In chloroform-d1; water at 20℃; |
-
-
1722-12-9
2-chloropyrimidine

-
-
23978-55-4
1,4,10,13-tetraoxa-7,16-diazacyclooctadecane

-
-
130536-58-2
7,16-Di-pyrimidin-2-yl-1,4,10,13-tetraoxa-7,16-diaza-cyclooctadecane

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 100℃; under 6000480 Torr; for 96h; | 100% |
-
-
1722-12-9
2-chloropyrimidine

-
-
1730-25-2
allylmagnesium bromide

-
-
123703-54-8
4-allyl-2-chloro-3,4-dihydropyrimidine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 0.25h; Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| With NmN'''-(butane-1,4-diyl)bisguanidine for 16h; Heating; | 100% |
-
-
1722-12-9
2-chloropyrimidine

-
-
594-19-4
tert.-butyl lithium

-
-
66522-06-3
4-(tert-butyl)-2-chloropyrimidine

| Conditions | Yield |
|---|---|
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In diethyl ether at -30 - 0℃; for 1h; | 100% |
| With acetic acid; bunazosin In tetrahydrofuran at -78℃; for 3h; |

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride; 2-aminopyrimidine-4,6-diol; copper(I) bromide at 145 - 150℃; for 24h; | 100% |
| With potassium carbonate In dimethyl sulfoxide at 70℃; for 24h; Inert atmosphere; | 95% |
| With 1-methyl-3-(2-pyridinyl)-3,4,5,6-tetrahydropyrimidin-3-ium hexafluorophosphate; potassium tert-butylate; palladium diacetate In 1,2-dimethoxyethane at 100℃; for 0.666667h; Buchwald-Hartwig Coupling; Microwave irradiation; | 93% |

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride; 2-aminopyrimidine-4,6-diol; copper(I) bromide at 145 - 150℃; for 24h; | 100% |
| With copper(I) oxide; 1,10-Phenanthroline; tetrabutyl ammonium fluoride at 140 - 145℃; for 24h; | 98% |
| Stage #1: 2-methylimidazole With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0℃; for 0.5h; Stage #2: 2-chloropyrimidine In N,N-dimethyl-formamide; mineral oil at 130℃; | 89% |
| With potassium phosphate; benzoin oxime; copper diacetate In dimethyl sulfoxide at 80℃; Inert atmosphere; | 80% |
| With copper; caesium carbonate; methyl-alpha-D-glucopyranoside In water; dimethyl sulfoxide at 110℃; for 14h; Sealed tube; Green chemistry; | 80% |
-
-
1722-12-9
2-chloropyrimidine

-
-
146140-95-6
2-pivaloylaminophenyl boronic acid

-
-
797047-16-6
2,2-dimethyl-N-(2-pyrimidin-2-yl-phenyl)-propionamide

| Conditions | Yield |
|---|---|
| Stage #1: 2-chloropyrimidine; tetrakis(triphenylphosphine) palladium(0) In glyco dimethyl ether at 15 - 25℃; for 0.333333 - 0.5h; Stage #2: 2-pivaloylaminophenyl boronic acid With sodium carbonate In 1,2-dimethoxyethane; water at 78 - 83℃; for 3h; Heating / reflux; | 100% |
| With sodium hydrogencarbonate; tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran; water at 20 - 83℃; | |
| With sodium carbonate; tetrakis(triphenylphosphine) palladium(0) In 1,2-dimethoxyethane; water at 0 - 83℃; for 4.33333 - 5.5h; Heating / reflux; | |
| With sodium hydrogencarbonate; tetrakis(triphenylphosphine) palladium(0) In 1,2-dimethoxyethane; water at 83℃; for 11h; |

| Conditions | Yield |
|---|---|
| With potassium carbonate; hydrazine hydrate at 100℃; for 0.5h; | 99.8% |
| With hydrazine In pyridine at 25℃; for 1.5h; | 95% |
| With hydrazine hydrate In ethanol Reflux; | 87% |

| Conditions | Yield |
|---|---|
| With potassium phosphate; bis(η3-allyl-μ-chloropalladium(II)); 1,2-bis(diphenylphosphino)-1'-(diisopropylphosphino)-3',4-di-tert-butyl ferrocene In toluene at 115℃; for 20h; Inert atmosphere; | 99% |
| With copper; caesium carbonate In N,N-dimethyl-formamide at 100℃; for 0.166667h; Ullmann ether synthesis; microwave irradiation; | 97% |
| With 2,2,6,6-tetramethylheptane-3,5-dione; caesium carbonate; copper(l) chloride In 1-methyl-pyrrolidin-2-one at 120℃; for 24h; Ullmann coupling; Inert atmosphere; | 65% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In neat (no solvent) at 160℃; for 3h; Schlenk technique; Inert atmosphere; | 99% |
| With copper(l) iodide; caesium carbonate In N,N-dimethyl-formamide at 20 - 100℃; for 24.5h; | 97% |
| With copper(l) iodide; caesium carbonate In N,N-dimethyl-formamide at 120℃; for 40h; | 97% |
-
-
1722-12-9
2-chloropyrimidine

-
-
4294-57-9
para-methylphenylmagnesium bromide

-
-
77232-13-4
2-p-tolyl-pyrimidine

| Conditions | Yield |
|---|---|
| With N-heterocyclic carbene-based nickel(II) complex In tetrahydrofuran at 20℃; for 12h; Kumada reaction; | 99% |
| With chloro(1,3-bis(pyridin-2-ylmethyl)imidazolylidene)nickel(II) hexafluorophosphate In tetrahydrofuran at 20℃; for 12h; Kumada-Corriu coupling reaction; Inert atmosphere; | 99% |
| With C16H16Cl2CoN8(1+)*F6P(1-) In tetrahydrofuran at 20℃; for 2h; Kumada-Corriu cross-coupling; | 97% |
| With (IPr)Ni(π-allyl)Cl In tetrahydrofuran at 20℃; for 24h; Kumada Cross-Coupling; Inert atmosphere; | 74% |
| With [Ni(1-(1-ethyl-benzimidazol-2-ylmethyl)-3-mesitylimidazolin-2-ylidene)2][Cl]2; sodium acetate In tetrahydrofuran at 20℃; Kumada-Corriu coupling reaction; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With C21H18N8Ni2O(2+)*2F6P(1-) In tetrahydrofuran; 1-methyl-pyrrolidin-2-one at 20 - 80℃; Negishi coupling reaction; | 99% |

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; sodium carbonate In 1,4-dioxane at 90℃; Suzuki coupling reaction; | 99% |
| With bis-triphenylphosphine-palladium(II) chloride; sodium carbonate In 1,4-dioxane at 90℃; Suzuki coupling; | 93% |
| Suzuki coupling; |
-
-
1722-12-9
2-chloropyrimidine

-
-
156-87-6
propan-1-ol-3-amine

-
-
959052-29-0
3-(pyrimidin-2-ylamino)propan-1-ol

| Conditions | Yield |
|---|---|
| In ethanol for 18h; Reflux; Inert atmosphere; | 99% |
| With triethylamine In butan-1-ol at 125℃; for 1h; Temperature; Inert atmosphere; Microwave irradiation; | 87% |
-
-
1722-12-9
2-chloropyrimidine

-
-
1414782-20-9
tert-butyl 2-[(R)-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2,3-dihydro-1H-inden-1-yl]-2,7-diazaspiro[3.5]nonane-7-carboxylate

-
-
1334785-04-4
tert-butyl 2-[(R)-5-(pyrimidin-2-yl)-2,3-dihydro-1H-inden-1-yl]-2,7-diazaspiro[3.5]nonane-7-carboxylate

| Conditions | Yield |
|---|---|
| With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium carbonate In 1,4-dioxane; water Suzuki Coupling; Inert atmosphere; Heating; | 99% |
| With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium carbonate In water at 100℃; for 18h; Suzuki Coupling; |

| Conditions | Yield |
|---|---|
| Stage #1: 2-(Trimethylsilyl)ethanol With sodium hydride In tetrahydrofuran at 0℃; for 1h; Stage #2: 2-chloropyrimidine In tetrahydrofuran at 25℃; for 24h; | 99% |
-
-
22428-87-1
4,4-ethylenedioxycyclohexan-1-ol

-
-
1722-12-9
2-chloropyrimidine

-
-
1361390-86-4
2-(1,4-dioxaspiro[4.5]decan-8-yloxy)pyrimidine

| Conditions | Yield |
|---|---|
| Stage #1: 4,4-ethylenedioxycyclohexan-1-ol With 1H-imidazole; sodium hydride In N,N-dimethyl acetamide; mineral oil at 20℃; for 0.5h; Cooling with ice; Stage #2: 2-chloropyrimidine In N,N-dimethyl acetamide; mineral oil at 20 - 60℃; for 3.5h; | 98.59% |
| Stage #1: 4,4-ethylenedioxycyclohexan-1-ol With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0 - 20℃; for 0.5h; Stage #2: 2-chloropyrimidine In N,N-dimethyl-formamide; mineral oil at 0 - 70℃; for 17h; | 97% |
| Stage #1: 4,4-ethylenedioxycyclohexan-1-ol With sodium hydride In N,N-dimethyl-formamide for 0.333333h; Stage #2: 2-chloropyrimidine at 20 - 100℃; for 9.5h; | |
| Stage #1: 4,4-ethylenedioxycyclohexan-1-ol With sodium hydride In N,N-dimethyl-formamide for 0.333333h; Stage #2: 2-chloropyrimidine In N,N-dimethyl-formamide at 100℃; for 9h; |

| Conditions | Yield |
|---|---|
| With cesium acetate; copper In dimethyl sulfoxide at 110℃; for 24h; Inert atmosphere; | 98% |
| at 100℃; for 0.1h; microwave irradiation; | 91% |
| With triethylamine In ethanol at 120℃; for 0.0833333h; Microwave irradiation; | 89% |
-
-
1722-12-9
2-chloropyrimidine

-
-
134896-34-7
(2-propoxyphenyl)boronic acid

-
-
134896-36-9
2-(2-Propoxyphenyl)pyrimidine

| Conditions | Yield |
|---|---|
| <1,4-bis-(diphenylphosphine)butane>palladium(II) | 98% |
| With sodium carbonate; [1,4-bis(diphenylphosphino)butane] palladium(ll) dichloride In ethanol; toluene for 24h; Heating; | 98% |
-
-
1722-12-9
2-chloropyrimidine

-
-
16419-60-6
2-Methylphenylboronic acid

-
-
188527-65-3
2-(2-methylphenyl)pyrimidine

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; sodium carbonate In 1,4-dioxane at 90℃; Suzuki coupling reaction; | 98% |
| With bis-triphenylphosphine-palladium(II) chloride; sodium carbonate In 1,4-dioxane at 90℃; Suzuki coupling; | 93% |
| With Tedicyp; potassium carbonate; bis(η3-allyl-μ-chloropalladium(II)) In xylene at 130℃; for 20h; Suzuki cross-coupling reaction; | 87% |
| Suzuki coupling; |
-
-
1722-12-9
2-chloropyrimidine

-
-
1334784-74-5
5-(pyrimidin-2-yl)-2,3-dihydro-1H-inden-1-one

| Conditions | Yield |
|---|---|
| With sodium carbonate; (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride In 1,2-dimethoxyethane; water at 20 - 95℃; Inert atmosphere; | 98% |
| With sodium carbonate In 1,2-dimethoxyethane at 95℃; Suzuki Coupling; Inert atmosphere; | 41% |

| Conditions | Yield |
|---|---|
| With bis(η3-allyl-μ-chloropalladium(II)); 1,1',3,3'-tetrakis(diphenylphosphino)ferrocene; sodium t-butanolate In toluene for 17h; Catalytic behavior; Inert atmosphere; Schlenk technique; Heating; | 98% |
| With fac-tris[2-phenylpyridinato-C2,N]iridium(III); caesium carbonate In N,N-dimethyl-formamide at 25℃; for 18h; Schlenk technique; Inert atmosphere; Irradiation; | 83% |
| Stage #1: 2-chloropyrimidine With tris-(dibenzylideneacetone)dipalladium(0); triethylamine In toluene at 115℃; for 1h; Inert atmosphere; Schlenk technique; Stage #2: thiophenol In toluene at 115℃; for 24h; Reagent/catalyst; Time; Inert atmosphere; Schlenk technique; | 72% |

| Conditions | Yield |
|---|---|
| With C50H61Cl2N3Pd; potassium tert-butylate In 1,4-dioxane at 100℃; for 2h; | 98% |
| With C48H55ClN2Pd; sodium t-butanolate In 1,2-dimethoxyethane at 20℃; for 16h; Inert atmosphere; Sealed tube; | 98% |
| With potassium carbonate In tetrahydrofuran for 2h; Reflux; | 44% |

| Conditions | Yield |
|---|---|
| With tris(dibenzylideneacetone)dipalladium(0) chloroform complex; zinc diacetate; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In 1,4-dioxane at 100℃; for 16h; Buchwald-Hartwig Coupling; Inert atmosphere; | 98% |
-
-
1722-12-9
2-chloropyrimidine

-
-
7378-99-6
N,N-dimethyloctanamide

-
A
-
5621-02-3
2-(dimethylamino)pyrimidine

-
B
-
141193-16-0
Methyl-octyl-pyrimidin-2-yl-amine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 100℃; under 6000480 Torr; for 96h; | A 2% B 97% |


| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; sodium carbonate In 1,4-dioxane at 90℃; Suzuki coupling reaction; | 97% |
| With bis-triphenylphosphine-palladium(II) chloride; sodium carbonate In 1,4-dioxane at 90℃; | 95% |
| With oxygen; palladium diacetate; sodium carbonate In 1,4-dioxane at 90℃; Suzuki Coupling; | 93% |
-
-
1722-12-9
2-chloropyrimidine

-
-
31575-35-6
2-fluoropyrimidine

| Conditions | Yield |
|---|---|
| With hydrogen fluoride at 50℃; for 1h; other aromatic halides, var. temp., also with HF-base systems; | 97% |
| With hydrogen fluoride at 50℃; for 1h; | 97% |
-
-
1722-12-9
2-chloropyrimidine

-
-
33941-15-0
1-aza-18-crown-6

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 100℃; under 6000480 Torr; for 96h; | 97% |

| Conditions | Yield |
|---|---|
| for 3h; Reflux; | 97% |
| at 20℃; for 2h; | 95% |
| for 2h; Heating; Yield given; |

| Conditions | Yield |
|---|---|
| In N,N,N,N,N,N-hexamethylphosphoric triamide at 85℃; for 0.00833333h; microwave irradiation; | 97% |
| In ethanol for 4h; Heating; | 14% |
2-Chloropyrimidine Chemical Properties
EINECS 217-020-2
Molecular Structure:
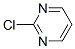
Melting point:66 oC
Boiling point:75-76 oC (10 mmHg)
Flash point:98 oC
Storage temp. : Refrigerator
Sensitive : Moisture Sensitive
2-Chloropyrimidine Uses
2-Chloropyrimidine Safety Profile
 Xi
Xi2-Chloropyrimidine's Risk Codes:
36/38:Irritating to eyes and skin
2-Chloropyrimidine's Safety Description:
26:In case of contact with eyes, rinse immediately with plenty of WATER and seek medical advice
37/39:Wear suitable gloves and eye/face protection
2-Chloropyrimidine Standards and Recommendations
Content:≥98%
Related Products
- 2-Chloropyrimidine
- 2-Chloropyrimidine-4,5-diamine
- 2-Chloropyrimidine-4-carboxylic acid
- 2-Chloropyrimidine-5-boronic acid
- 2-Chloropyrimidine-5-carbaldehyde
- 2-Chloropyrimidine-5-carboxylic acid
- 2-Chloropyrimidinehydrochloride
- 1722-14-1
- 172217-11-7
- 172217-16-2
- 1722-18-5
- 172222-30-9
- 172223-58-4
- 17222-56-9
- 1722-26-5
- 172256-07-4
- 17225-95-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View