-
Name
2-Hydroxynicotinic acid
- EINECS 210-198-2
- CAS No. 609-71-2
- Article Data47
- CAS DataBase
- Density 1.451 g/cm3
- Solubility
- Melting Point 258-261 °C(lit.)
- Formula C6H5NO3
- Boiling Point 436 °C at 760 mmHg
- Molecular Weight 139.111
- Flash Point 217.5 °C
- Transport Information
- Appearance white to light yellow powder
- Safety 26-36-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 1,2-Dihydro-2-oxonicotinic acid;2-Hydroxy-3-pyridinecarboxylic acid;2-Hydroxy-3-Pyridine Carboxylic acid;2-oxo-1H-pyridine-3-carboxylic acid;2-oxo-1H-pyridine-3-carboxylate;1,2-Dihydro-2-oxo-3-pyridinecarboxylic acid;
- PSA 70.42000
- LogP 0.48540
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water for 6h; Heating; | 92% |
| With copper(I) oxide; 2-pyridinealdoxime; cesium hydroxide; tetrabutylammomium bromide In water at 110℃; for 48h; Inert atmosphere; | 63% |
| Heating; | |
| With sodium hydroxide In ethanol; water |

| Conditions | Yield |
|---|---|
| Stage #1: 3-bromo-pyridin-2-ol With isopropylmagnesium chloride In tetrahydrofuran at 0℃; for 0.166667h; Stage #2: With n-butyllithium In tetrahydrofuran; hexane at -20℃; for 0.5h; Stage #3: carbon dioxide In tetrahydrofuran; hexane at -20℃; for 0.5h; | 79% |
-
-
2398-81-4
Nicotinic acid N-oxide

-
-
108-24-7
acetic anhydride

-
A
-
5006-66-6
6-hydroxy-3-pyridinecarboxylic acid

-
B
-
609-71-2
2-hydroxy-3-carboxypyridine

-
C
-
111068-01-0
3-acetoxy-4-aza-3-methyl-1(3H)-isobenzofuranone 4-oxide

| Conditions | Yield |
|---|---|
| for 6h; Heating; | A 1.5% B 5.4% C 62% |

-
-
2398-81-4
Nicotinic acid N-oxide

-
A
-
5006-66-6
6-hydroxy-3-pyridinecarboxylic acid

-
B
-
609-71-2
2-hydroxy-3-carboxypyridine

-
C
-
111068-01-0
3-acetoxy-4-aza-3-methyl-1(3H)-isobenzofuranone 4-oxide

| Conditions | Yield |
|---|---|
| With acetic anhydride for 6h; Heating; | A 1.5% B 5.4% C 62% |

-
-
2942-59-8
2-chloronicotinic acid

-
A
-
609-71-2
2-hydroxy-3-carboxypyridine

-
B
-
5345-47-1
2-aminopyridin-3-carboxylic acid

| Conditions | Yield |
|---|---|
| With ammonium hydroxide at 155℃; for 20h; | A n/a B 60% |
-
-
83164-33-4
N-(2,4-difluorophenyl)-2-(3-trifluoromethylphenoxy)-3-pyridinecarboxamide

-
A
-
609-71-2
2-hydroxy-3-carboxypyridine

-
B
-
98-17-9
3-Trifluoromethylphenol

-
C
-
367-25-9
2,4-difluorophenylamine

-
D
-
130191-66-1
N-(2,4-Difluorophenyl)-2-hydroxy-3-pyridinecarboxamide

| Conditions | Yield |
|---|---|
| With hydrogenchloride; acetic acid In water for 48h; Heating; | A 57% B n/a C n/a D 28% |
| With hydrogenchloride; acetic acid In water for 48h; Product distribution; Mechanism; Heating; | A 57% B n/a C n/a D 28% |

-
-
2398-81-4
Nicotinic acid N-oxide

-
-
123-62-6
propionic acid anhydride

-
A
-
609-71-2
2-hydroxy-3-carboxypyridine

-
C
-
115147-79-0
5-aza-3-methyl-4-propionyloxyisocoumarin

| Conditions | Yield |
|---|---|
| at 125℃; for 6h; | A n/a B n/a C 12.9% D 54.2% |

-
-
2398-81-4
Nicotinic acid N-oxide

-
-
123-62-6
propionic acid anhydride

-
A
-
609-71-2
2-hydroxy-3-carboxypyridine

-
C
-
115147-79-0
5-aza-3-methyl-4-propionyloxyisocoumarin

-
D
-
115147-80-3
4-aza-3-ethyl-3-propionyloxy-1(3H)-isobenzofuranone

| Conditions | Yield |
|---|---|
| at 160℃; for 4h; Yield given. Further byproducts given; | A n/a B n/a C 39.8% D 16.4% |

-
-
2398-81-4
Nicotinic acid N-oxide

-
-
123-62-6
propionic acid anhydride

-
A
-
609-71-2
2-hydroxy-3-carboxypyridine

-
C
-
115147-79-0
5-aza-3-methyl-4-propionyloxyisocoumarin

-
D
-
115147-80-3
4-aza-3-ethyl-3-propionyloxy-1(3H)-isobenzofuranone

-
F
-
115147-81-4
4-aza-3-ethyl-3,5-dipropionyloxy-1(3H)-isobenzofuranone

| Conditions | Yield |
|---|---|
| at 160℃; for 4h; Product distribution; Mechanism; various temperatures and times; | A n/a B n/a C 39.8% D 16.4% E 8.3% F 2.1% |

-
-
504-29-0
2-aminopyridine

-
-
2942-59-8
2-chloronicotinic acid

-
A
-
609-71-2
2-hydroxy-3-carboxypyridine

-
B
-
99973-09-8
2-hydroxy-nicotinic acid-[2]pyridylamide

-
C
-
109255-91-6
2-(2-hydroxy-nicotinoylimino)-2H-[1,2']bipyridyl-3'-carboxylic acid

| Conditions | Yield |
|---|---|
| at 180 - 185℃; |

-
-
20577-27-9
2-hydroxy-3-cyanopyridine

-
-
609-71-2
2-hydroxy-3-carboxypyridine

| Conditions | Yield |
|---|---|
| With hydrogenchloride |

| Conditions | Yield |
|---|---|
| With hydrogenchloride |
-
-
35905-85-2
2-bromonicotinic acid

-
-
609-71-2
2-hydroxy-3-carboxypyridine

| Conditions | Yield |
|---|---|
| With potassium hydroxide; water at 180℃; |
-
-
10128-92-4
2-hydroxynicotinamide

-
-
609-71-2
2-hydroxy-3-carboxypyridine
-
-
59-67-6
nicotinic acid

-
A
-
5006-66-6
6-hydroxy-3-pyridinecarboxylic acid

-
B
-
609-71-2
2-hydroxy-3-carboxypyridine

| Conditions | Yield |
|---|---|
| With potassium hydroxide; fluorine In water at 0℃; for 3.5h; Yield given. Yields of byproduct given; | |
| With potassium hydroxide; fluorine In water at 0℃; Yield given. Yields of byproduct given; | |
| With water; oxygen Quantum yield; Further Variations:; pH-values; Reagents; Irradiation; |
-
-
91-22-5
quinoline

-
A
-
500-22-1
3-pyridinecarboxaldehyde

-
B
-
59-67-6
nicotinic acid

-
C
-
609-71-2
2-hydroxy-3-carboxypyridine

-
D
-
59-31-4
2-hydroxyquinoline

| Conditions | Yield |
|---|---|
| With oxygen at 260 - 280℃; under 15751.3 - 64505.2 Torr; pH=7; Kinetics; Further Variations:; pH-values; Temperatures; Oxidation; |


-
-
609-71-2
2-hydroxy-3-carboxypyridine

| Conditions | Yield |
|---|---|
| With sulfuric acid; sodium nitrite Diazotization; |

-
-
609-71-2
2-hydroxy-3-carboxypyridine

| Conditions | Yield |
|---|---|
| With Pd-BaSO4; ethanol Hydrogenation; |

-
-
609-71-2
2-hydroxy-3-carboxypyridine

| Conditions | Yield |
|---|---|
| With acetic anhydride; acetic acid at 210℃; im Rohr; |

-
A
-
609-71-2
2-hydroxy-3-carboxypyridine

-
B
-
10349-57-2
Quinoline-6-carboxylic acid

| Conditions | Yield |
|---|---|
| With potassium permanganate; sulfuric acid |
-
-
504-29-0
2-aminopyridine

-
-
2942-59-8
2-chloronicotinic acid

-
A
-
609-71-2
2-hydroxy-3-carboxypyridine

-
B
-
99973-09-8
2-hydroxy-nicotinic acid-[2]pyridylamide

| Conditions | Yield |
|---|---|
| at 180 - 185℃; |


| Conditions | Yield |
|---|---|
| With sulfuric acid; quinolinium dichromate(VI); acetic acid at 50℃; for 24h; Kinetics; | |
| With sulfuric acid; quinolinium dichromate(VI); acetic acid In water at 49.85℃; for 48h; Kinetics; Further Variations:; Solvents; Temperatures; | |
| With quinolinium dichromate In water; acetic acid at 49.84℃; for 48h; Kinetics; Mechanism; Solvent; Temperature; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 80 percent / 30percent H2O2 / acetic acid / 3 h / 90 °C 2: PCl5, POCl3 3: conc. NH4OH / 20 h / 155 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 80 percent / 30percent H2O2 / acetic acid / 3 h / 90 °C 2: 65 percent / POCl3, Et3N / 4 h / 100 °C 3: conc. NH4OH / 20 h / 155 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: PCl5, POCl3 2: conc. NH4OH / 20 h / 155 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 65 percent / POCl3, Et3N / 4 h / 100 °C 2: conc. NH4OH / 20 h / 155 °C View Scheme | |
| Multi-step reaction with 2 steps 1: POCl3 / dimethylformamide 2: Heating View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: PCl5; POCl3 / 115 - 120 °C 2: aqueous HCl View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: CH3I / 80 °C / Behandeln des Reaktionsprodukts mit H2O View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: aqueous H2O2; acetic acid 2: PCl5; POCl3 / 115 - 120 °C 3: aqueous HCl View Scheme |
-
-
100921-66-2
bromo-2 butyl-3 pyridine

-
-
609-71-2
2-hydroxy-3-carboxypyridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: KMnO4; H2O 2: KOH; H2O / 180 °C View Scheme |
-
-
609-71-2
2-hydroxy-3-carboxypyridine

-
-
49609-84-9
2-Chloronicotinoyl chloride

| Conditions | Yield |
|---|---|
| With oxalyl dichloride In dichloromethane; N,N-dimethyl-formamide at 20℃; for 4h; Inert atmosphere; | 100% |
| With phosphorus pentachloride anschliessendes Erwaermen; | |
| With phosphorus pentachloride anschliessendes Erwaermen; |
-
-
609-71-2
2-hydroxy-3-carboxypyridine

-
-
999-97-3
1,1,1,3,3,3-hexamethyl-disilazane

-
-
183274-22-8
trimethylsilyl 2-(trimethylsilyloxy)nicotinate

| Conditions | Yield |
|---|---|
| at 150℃; for 7h; | 100% |
| With chloro-trimethyl-silane In toluene Heating; |

| Conditions | Yield |
|---|---|
| With acetyl chloride at 0 - 20℃; | 100% |
-
-
67-56-1
methanol

-
-
609-71-2
2-hydroxy-3-carboxypyridine

-
-
10128-91-3
methyl-2-oxo-1,2-dihydro-3-pyridinecarbonitrile

| Conditions | Yield |
|---|---|
| With sulfuric acid Heating / reflux; | 100% |
| Stage #1: 2-hydroxy-3-carboxypyridine With thionyl chloride In dichloromethane at 0℃; Stage #2: In tetrahydrofuran; dichloromethane at 20℃; Stage #3: methanol In tetrahydrofuran; dichloromethane at 20℃; | 81.8% |
| With sulfuric acid In toluene Dean-Stark; Reflux; | 74% |
-
-
67-56-1
methanol

-
-
609-71-2
2-hydroxy-3-carboxypyridine

-
-
203937-66-0
methyl 2-oxo-1,2-dihydropyridine-3-carboxylate hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: 2-hydroxy-3-carboxypyridine With thionyl chloride In tetrahydrofuran; dichloromethane at 20℃; for 1h; Stage #2: methanol In tetrahydrofuran; dichloromethane at 20℃; | 100% |
-
-
609-71-2
2-hydroxy-3-carboxypyridine

-
-
7732-18-5
water

-
-
6147-53-1
cobalt(II) diacetate tetrahydrate

-
-
956331-98-9
diaquabis(2-hydroxynicotinato(1-))cobalt(II)

| Conditions | Yield |
|---|---|
| With aq. ammonia In water soln. of ligand in H2O added to soln. of Co salt in H2O; pH adjusted to 8 (a few drops of concd. ammonia soln.); stored for a few d; filtered; ppt. washed with H2O; dried in dessicator over NaOH; elem. anal.; | 100% |

-
-
609-71-2
2-hydroxy-3-carboxypyridine

-
-
14871-92-2
dichloro(2,2'-bipyridine)palladium(II)

-
-
503811-68-5
[Pd bis(2-hydroxynicotinate)(2,2'-bipyridine)

| Conditions | Yield |
|---|---|
| With triethylamine In methanol; ethanol stirred suspn. of Pd complex in methanol mixed with methanolic suspn. ofligand and triethylamine, stirred for 1 day; sentrifuged, washed with ethanol, dried (silica gel); elem. anal.; | 99% |
-
-
609-71-2
2-hydroxy-3-carboxypyridine

-
-
530-62-1
1,1'-carbonyldiimidazole

-
-
211425-43-3
(2-Hydroxy-pyridin-3-yl)-imidazol-1-yl-methanone

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 19h; Heating; | 98% |
| In tetrahydrofuran for 24h; Heating; | |
| With Imidazole hydrochloride In tetrahydrofuran at 50℃; for 4h; |
-
-
89430-08-0
C20H15N2PS2

-
-
609-71-2
2-hydroxy-3-carboxypyridine

| Conditions | Yield |
|---|---|
| In dichloromethane | 97% |
-
-
609-71-2
2-hydroxy-3-carboxypyridine

-
-
1416767-53-7
5,5-dibenzyl-2,3,4,5-tetrahydropyridine

-
-
1416767-45-7
10,10-dibenzyl-8,9,10,10a-tetrahydrodipyrido[2,1-b:3',2'-e][1,3]oxazin-5(7H)-one

| Conditions | Yield |
|---|---|
| With 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide; N-ethyl-N,N-diisopropylamine In toluene at 90℃; for 20h; Inert atmosphere; | 97% |
| With 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide; N-ethyl-N,N-diisopropylamine In toluene at 90℃; for 20h; | 97% |
-
-
609-71-2
2-hydroxy-3-carboxypyridine

-
-
74-88-4
methyl iodide

-
-
15506-18-0
1-methyl-2-oxo-1,2-dihydropyridine-3-carboxylic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol; water for 2h; Methylation; Heating; | 96% |
| With potassium hydroxide In methanol; water for 12h; Reflux; Inert atmosphere; | 65% |
| With potassium hydroxide In methanol; water at 0 - 80℃; for 16h; | 45% |
-
-
609-71-2
2-hydroxy-3-carboxypyridine

-
-
352-11-4
1-chloromethyl-4-fluorobenzene

-
-
66158-41-6
1,2-dihydro-1-(4-fluorobenzyl)-2-oxopyridine-3-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: 2-hydroxy-3-carboxypyridine With sodium hydride In N,N-dimethyl-formamide at 20℃; for 1h; Stage #2: 1-chloromethyl-4-fluorobenzene In N,N-dimethyl-formamide at 50℃; for 12h; Stage #3: With sodium hydroxide In water for 4h; Reflux; | 96% |
-
-
609-71-2
2-hydroxy-3-carboxypyridine

-
-
38076-80-1
5-chloro-2-hydroxypyridine-3-carboxylic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium hydroxide; sodium hypochlorite; chlorine; sodium hydrogensulfite In water; acetone | 95.6% |
| With sodium hydroxide; chlorine In water at 0 - 35℃; | 84% |
| Stage #1: 2-hydroxy-3-carboxypyridine With sodium hydroxide; sodium hypochlorite In water at 20℃; for 48h; Stage #2: With sodium sulfite In water at 20℃; for 0.5h; | 78% |
-
-
609-71-2
2-hydroxy-3-carboxypyridine

-
-
42463-41-2
3-(hydroxymethyl)pyridin-2(1H)-one

| Conditions | Yield |
|---|---|
| Stage #1: 2-hydroxy-3-carboxypyridine With chloro-trimethyl-silane; 1,1,1,2,2,2-hexamethyldisilane In toluene at 110℃; for 0.5h; Stage #2: With diisobutylaluminium hydride In dichloromethane; toluene at -40 - 20℃; Stage #3: With hydrogenchloride; water In dichloromethane; toluene at -10℃; pH=4; | 95% |
| Stage #1: 2-hydroxy-3-carboxypyridine With chloro-trimethyl-silane; 1,1,1,3,3,3-hexamethyl-disilazane In toluene for 2h; Heating / reflux; Stage #2: With diisobutylaluminium hydride In toluene at -78℃; for 4h; Stage #3: With methanol In toluene | 59% |
-
-
67-56-1
methanol

-
-
609-71-2
2-hydroxy-3-carboxypyridine

-
-
28966-81-6
trans-bis(triphenylphosphine)palladium dichloride

| Conditions | Yield |
|---|---|
| With triethylamine In methanol; ethanol stirred suspn. of Pd complex mixed with methanolic suspn. of ligand and triethylamine, stirred for 1 week; centrifuged, washed with ethanol, dried (silica gel); elem. anal., XRD; | 94% |

-
-
609-71-2
2-hydroxy-3-carboxypyridine

-
-
100-39-0
benzyl bromide

-
-
89960-36-1
1-benzyl-2-oxo-1,2-dihydropyridine-3-carboxylic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol; water for 8.5h; Heating; | 93% |
| With potassium hydroxide In methanol; water for 12h; Reflux; Inert atmosphere; | 71% |
| Stage #1: 2-hydroxy-3-carboxypyridine With potassium hydroxide In methanol; water for 0.25h; Heating / reflux; Stage #2: benzyl bromide In methanol; water for 1.5h; Heating / reflux; Stage #3: With hydrogenchloride; water | 55% |
-
-
609-71-2
2-hydroxy-3-carboxypyridine

-
-
111409-79-1
1-bromo-2-(triisopropylsilyl)acetylene

| Conditions | Yield |
|---|---|
| With [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; potassium carbonate at 90℃; for 14h; | 92% |

| Conditions | Yield |
|---|---|
| In water ligand added to soln. of Mo salt, suspn. refluxed for 6 h; filtered, washed with water, dried (silica gel); | 91% |

-
-
609-71-2
2-hydroxy-3-carboxypyridine

-
-
371-40-4
4-fluoroaniline

-
-
951314-43-5
N-(4-fluorophenyl)-2-hydroxynicotinamide

| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane Reflux; | 91% |
-
-
609-71-2
2-hydroxy-3-carboxypyridine

-
-
20989-17-7
(2S)-2-phenylglycinol

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at -15 - 20℃; Inert atmosphere; | 90% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-hydroxy-3-carboxypyridine With 1,1'-carbonyldiimidazole In tetrahydrofuran at 70℃; for 24h; Stage #2: diethylamine In tetrahydrofuran at 70℃; for 18h; | 90% |
| Stage #1: 2-hydroxy-3-carboxypyridine With 1,1'-carbonyldiimidazole In tetrahydrofuran at 60℃; for 1h; Inert atmosphere; Stage #2: diethylamine In tetrahydrofuran at 60℃; for 2h; Inert atmosphere; | 85% |

| Conditions | Yield |
|---|---|
| With NaOMe In methanol byproducts: NaCl; suspn. of Ru-complex and ligand in MeOH was stirred at room temp. for 10min, soln. of NaOMe in MeOH was added, stirred for 30 min under N2; solvent was evapd. under reduced pressure, extd. with CH2Cl2, hexane wasadded, solvent was evapd.; elem. anal.; | 89% |


| Conditions | Yield |
|---|---|
| With sulfuric acid; nitric acid at 0 - 50℃; for 4h; | 88% |
| With sulfuric acid; nitric acid 1.) RT, 16 h, 2.) from 50 deg C to 70 deg C, 1.5 h; | 87% |
| With sulfuric acid; nitric acid | 87% |

| Conditions | Yield |
|---|---|
| With n-Bu4POH In tetrahydrofuran; water at 0 - 20℃; | 88% |
| Stage #1: 2-hydroxy-3-carboxypyridine; benzyl bromide With caesium carbonate In N,N-dimethyl-formamide at 20℃; for 24h; Inert atmosphere; Stage #2: With sodium hydroxide Inert atmosphere; |
-
-
2038-03-1
4-(2-AMINOETHYL)MORPHOLINE

-
-
609-71-2
2-hydroxy-3-carboxypyridine

-
-
1221489-81-1
2-hydroxy-N-(2-morpholinoethyl)nicotinamide

| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane Reflux; | 88% |
-
-
609-71-2
2-hydroxy-3-carboxypyridine

-
-
106-47-8
4-chloro-aniline

-
-
951314-44-6
N-(4-chlorophenyl)-2-hydroxynicotinamide

| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane Reflux; | 88% |
-
-
609-71-2
2-hydroxy-3-carboxypyridine

-
-
104612-36-4
5-bromo-2-hydroxynicotinic acid

| Conditions | Yield |
|---|---|
| Stage #1: With sodium hydroxide; bromine In water at 0℃; for 0.0833333h; Stage #2: 2-hydroxy-3-carboxypyridine With sodium hydroxide In water at 50℃; for 44h; Stage #3: With hydrogenchloride In water at 0℃; pH=2; | 87% |
| With sodium hydroxide; bromine at 0 - 50℃; for 18h; | 78% |
| With bromine In water; acetic acid | 78% |
-
-
609-71-2
2-hydroxy-3-carboxypyridine

-
-
13965-31-6
dichloro(2,2'-bipyridine)platinum(II)

-
-
503811-71-0
[PtCl(2-hydroxynicotinate)(2,2'-bipyridine)

| Conditions | Yield |
|---|---|
| With triethylamine In methanol; ethanol stirred suspn. of Pt complex in methanol mixed with methanolic suspn. ofligand and triethylamine, stirred for 2 weeks; sentrifuged, washed with ethanol, dried (silica gel); elem. anal.; | 87% |
-
-
609-71-2
2-hydroxy-3-carboxypyridine

-
-
106-49-0
p-toluidine

-
-
72646-01-6
2-hydroxy-N-(p-tolyl)nicotinamide

| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane Reflux; | 87% |
2-Hydroxynicotinic acid Chemical Properties
Molecular Structure of 2-Hydroxynicotinic acid (CAS NO. 609-71-2):
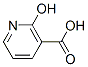
IUPAC Name: 2-Oxo-1H-pyridine-3-carboxylic acid
Molecular Formula: C6H5NO3
Molecular Weight: 139.108800 g/mol
Index of Refraction: 1.579
Molar Refractivity: 31.84 cm3
Molar Volume: 95.8 cm3
Surface Tension: 61 dyne/cm
Density: 1.451 g/cm3
Flash Point: 217.5 °C
Enthalpy of Vaporization: 75.92 kJ/mol
Boiling Point: 436 °C at 760 mmHg
Vapour Pressure: 8.03E-09 mmHg at 25 °C
Melting Point: 258-261 °C(lit.)
BRN: 119028
EINECS: 210-198-2
Product Categories: blocks;Carboxes;Pyridines;Pyridine;Pyridines, Pyrimidines, Purines and Pteredines;Organic acids;CHIRAL CHEMICALS
2-Hydroxynicotinic acid Safety Profile
Safety Information of 2-Hydroxynicotinic acid (CAS NO. 609-71-2):
Hazard Codes: Xi 
Hazard Note: Irritant
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin
Safety Statements: 26-36-37/39
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
S36:Wear suitable protective clothing
S37/39:Wear suitable gloves and eye/face protection
WGK Germany: 3
HazardClass: IRRITANT
2-Hydroxynicotinic acid Specification
2-Hydroxynicotinic acid with cas registry number of 609-71-2 is also called NSC 226152 ; 1,2-Dihydro-2-oxo-3-pyridinecarboxylic acid ; 1,2-Dihydro-2-oxonicotinic acid .
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View