-
Name
2-Hydroxypyridine
- EINECS 205-520-3
- CAS No. 142-08-5
- Article Data196
- CAS DataBase
- Density 1.111 g/cm3
- Solubility Soluble in water 450 g/L @ 20°C and ethanol, slightly soluble in benzene and ether.
- Melting Point 105-107 °C(lit.)
- Formula C5H5NO
- Boiling Point 323.7 °C at 760 mmHg
- Molecular Weight 95.1008
- Flash Point 181.1 °C
- Transport Information UN 2811
- Appearance white to light yellow crystal
- Safety 26-36-37/39-22
- Risk Codes 36/37/38-40
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, Xi
Xi
- Synonyms 2(1H)-Pyridone;1H-pyridin-2-one;2-Pyridone;2-Pyridinone;2-Pyridinol;2-Hydroxy pyridine;Pyridin-2(1H)-one;
- PSA 33.12000
- LogP 0.78720
Synthetic route
-
-
13161-30-3
2-hydroxy-pyridine N-oxide

-
-
142-08-5
2-hydroxypyridin

| Conditions | Yield |
|---|---|
| With triphenylphosphine; N-fused tetraphenylporphyrin rhenium(VII) trioxide In dichloromethane at 23℃; for 3h; | 99% |
| With carbon dioxide; water; iron at 100℃; for 10h; Autoclave; Green chemistry; chemoselective reaction; | 99% |
| With ammonium formate; silica gel; zinc In methanol at 20℃; for 0.166667h; chemoselective reaction; | 91% |

| Conditions | Yield |
|---|---|
| With triethylamine In water; N,N-dimethyl-formamide for 8h; Ambient temperature; | A n/a B 98% |

-
-
89985-91-1
tert-butyl pyridin-2-yl carbonate

-
-
147-85-3
L-proline

-
A
-
142-08-5
2-hydroxypyridin

-
B
-
15761-39-4
1-(tert-butoxycarbonyl)-L-proline

| Conditions | Yield |
|---|---|
| With triethylamine In water; N,N-dimethyl-formamide for 6h; Ambient temperature; | A n/a B 98% |

-
-
89985-91-1
tert-butyl pyridin-2-yl carbonate

-
-
73-22-3
L-Tryptophan

-
A
-
142-08-5
2-hydroxypyridin

-
B
-
13139-14-5
Boc-Trp-OH

| Conditions | Yield |
|---|---|
| With triethylamine In water; N,N-dimethyl-formamide for 4h; Ambient temperature; | A n/a B 98% |

-
-
89985-91-1
tert-butyl pyridin-2-yl carbonate

-
A
-
142-08-5
2-hydroxypyridin

-
B
-
2488-15-5
N-tert-butoxycarbonyl-L-methionine

| Conditions | Yield |
|---|---|
| With triethylamine In water; N,N-dimethyl-formamide for 7h; Ambient temperature; | A n/a B 96% |

-
-
89985-91-1
tert-butyl pyridin-2-yl carbonate

-
A
-
142-08-5
2-hydroxypyridin

-
B
-
13734-34-4
N-tert-butoxycarbonyl-L-phenylalanine

| Conditions | Yield |
|---|---|
| With triethylamine In water; N,N-dimethyl-formamide for 12h; Ambient temperature; | A n/a B 96% |

-
-
197958-29-5
pyridin-2-ylboronic acid

-
-
142-08-5
2-hydroxypyridin

| Conditions | Yield |
|---|---|
| With hydrazine hydrate; caesium carbonate In water at 80℃; for 12h; | 96% |
-
-
40864-08-2
2-benzyloxypyridine

-
-
142-08-5
2-hydroxypyridin

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine; tetrahydroxydiboron; 5%-palladium/activated carbon In 1,2-dichloro-ethane at 50℃; for 1h; | 96% |
| With [4,4’-bis(1,1-dimethylethyl)-2,2’-bipyridine-N1,N1‘]bis [3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl-N]phenyl-C]iridium(III) hexafluorophosphate; ascorbic acid In water; acetonitrile at 20℃; for 3h; Schlenk technique; Irradiation; Inert atmosphere; chemoselective reaction; | 95% |
-
-
67-56-1
methanol

-
-
117-34-0
2,2-diphenylacetic acid

-
A
-
142-08-5
2-hydroxypyridin

-
B
-
3469-00-9
methyl 2,2-diphenylacetate

| Conditions | Yield |
|---|---|
| With 2,2'-dipyridyl carbonate; dmap In dichloromethane for 1h; Ambient temperature; | A 90% B 95% |

-
-
134-32-7
1-amino-naphthalene

-
-
96989-50-3
di-2-pyridyl thionocarbonate

-
A
-
142-08-5
2-hydroxypyridin

-
B
-
551-06-4
1-isothiocyanatonaphthalene

| Conditions | Yield |
|---|---|
| In dichloromethane for 0.166667h; Ambient temperature; | A n/a B 95% |

-
-
96989-50-3
di-2-pyridyl thionocarbonate

-
-
106-49-0
p-toluidine

-
A
-
142-08-5
2-hydroxypyridin

-
B
-
622-59-3
1-isothiocyanato-4-methylbenzene

| Conditions | Yield |
|---|---|
| In dichloromethane for 0.0833333h; Ambient temperature; | A n/a B 95% |

-
-
108-98-5
thiophenol

-
-
621-82-9
Cinnamic acid

-
A
-
142-08-5
2-hydroxypyridin

-
B
-
70030-52-3
(E)-S-phenyl 3-phenylprop-2-enethioate

| Conditions | Yield |
|---|---|
| With 2,2'-dipyridyl carbonate; dmap In dichloromethane for 6h; Ambient temperature; | A 90% B 95% |

-
-
61-90-5
L-leucine

-
-
89985-91-1
tert-butyl pyridin-2-yl carbonate

-
A
-
142-08-5
2-hydroxypyridin

-
B
-
13139-15-6
N-tert-butoxycarbonyl-L-leucine

| Conditions | Yield |
|---|---|
| With triethylamine In water; N,N-dimethyl-formamide for 4h; Ambient temperature; | A n/a B 94% |

-
-
96989-50-3
di-2-pyridyl thionocarbonate

-
-
109-73-9
N-butylamine

-
A
-
142-08-5
2-hydroxypyridin

-
B
-
592-82-5
butyl isothiocyanate

| Conditions | Yield |
|---|---|
| In dichloromethane for 0.0833333h; Ambient temperature; | A n/a B 94% |

-
-
124-07-2
Octanoic acid

-
-
100-51-6
benzyl alcohol

-
A
-
142-08-5
2-hydroxypyridin

-
B
-
10276-85-4
benzyl caprylate

| Conditions | Yield |
|---|---|
| With 2,2'-dipyridyl carbonate; dmap In dichloromethane for 2h; Ambient temperature; | A 89% B 94% |


| Conditions | Yield |
|---|---|
| With 2,2'-dipyridyl carbonate; dmap In dichloromethane for 2h; Ambient temperature; | A 90% B 93% |

-
-
89985-91-1
tert-butyl pyridin-2-yl carbonate

-
A
-
142-08-5
2-hydroxypyridin

-
B
-
3262-72-4
(S)-N-(tert-butoxycarbonyl)serine

| Conditions | Yield |
|---|---|
| With triethylamine In water; N,N-dimethyl-formamide for 7h; Ambient temperature; | A n/a B 93% |

-
-
120-57-0
piperonal

-
-
1219454-89-3
2-((difluoromethyl)sulfinyl)pyridine

-
A
-
142-08-5
2-hydroxypyridin

-
B
-
253266-72-7
5-(2,2-difluorovinyl)benzo[d][1,3]dioxole

| Conditions | Yield |
|---|---|
| Stage #1: piperonal; 2-((difluoromethyl)sulfonyl)pyridine With potassium tert-butylate In N,N-dimethyl-formamide at -50 - -40℃; for 0.25h; Julia-Kocienski type gem-difluoroolefination; Inert atmosphere; Stage #2: With hydrogenchloride; water; ammonium chloride In N,N-dimethyl-formamide at -40 - 20℃; Inert atmosphere; | A n/a B 93% |

-
-
72-18-4
L-valine

-
-
89985-91-1
tert-butyl pyridin-2-yl carbonate

-
A
-
142-08-5
2-hydroxypyridin

-
B
-
13734-41-3
t-Boc-L-valine

| Conditions | Yield |
|---|---|
| With triethylamine In water; N,N-dimethyl-formamide for 9h; Ambient temperature; | A n/a B 92% |

-
-
4041-95-6
N,N-di-tert-butylthiourea

-
A
-
142-08-5
2-hydroxypyridin

-
B
-
691-24-7
1,3-di-tert-butylcarbodiimide

| Conditions | Yield |
|---|---|
| With di-2-pyridyl thionocarbonate; dmap In acetonitrile for 6h; Ambient temperature; | A n/a B 92% |

| Conditions | Yield |
|---|---|
| With 2,2'-dipyridyl carbonate; dmap In dichloromethane for 1h; Ambient temperature; | A 90% B 92% |

-
-
1219454-89-3
2-((difluoromethyl)sulfinyl)pyridine

-
-
66-99-9
β-naphthaldehyde

-
A
-
142-08-5
2-hydroxypyridin

-
B
-
131581-40-3
1,1-difluoro-2-(2-naphthyl)ethene

| Conditions | Yield |
|---|---|
| Stage #1: 2-((difluoromethyl)sulfonyl)pyridine; β-naphthaldehyde With potassium tert-butylate In N,N-dimethyl-formamide at -50 - -40℃; for 0.25h; Julia-Kocienski type gem-difluoroolefination; Inert atmosphere; Stage #2: With hydrogenchloride; water; ammonium chloride In N,N-dimethyl-formamide at -40 - 20℃; Inert atmosphere; | A n/a B 92% |

-
-
115-20-8
1,1,1-trichloroethanol

-
-
124-07-2
Octanoic acid

-
A
-
142-08-5
2-hydroxypyridin

-
B
-
84443-53-8
n-heptanoic acid 2,2,2-trichloroethylester

| Conditions | Yield |
|---|---|
| With 2,2'-dipyridyl carbonate; dmap In dichloromethane for 0.5h; Ambient temperature; | A 90% B 91% |

-
-
6336-01-2
3-n-butyl-1-phenylthiourea

-
A
-
142-08-5
2-hydroxypyridin

-
B
-
21848-95-3
N-phenyl-N'-(n-butyl)carbodiimide

| Conditions | Yield |
|---|---|
| With di-2-pyridyl thionocarbonate; dmap In acetonitrile for 1h; Ambient temperature; | A n/a B 91% |
-
-
1219454-89-3
2-((difluoromethyl)sulfinyl)pyridine

-
-
100-10-7
4-dimethylamino-benzaldehyde

-
A
-
142-08-5
2-hydroxypyridin

-
B
-
38935-94-3
4-dimethylamino-β,β-difluorostyrene

| Conditions | Yield |
|---|---|
| Stage #1: 2-((difluoromethyl)sulfonyl)pyridine; 4-dimethylamino-benzaldehyde With potassium tert-butylate In N,N-dimethyl-formamide at -50 - -40℃; for 0.25h; Julia-Kocienski type gem-difluoroolefination; Inert atmosphere; Stage #2: With hydrogenchloride; water; ammonium chloride In N,N-dimethyl-formamide at -40 - 20℃; Inert atmosphere; | A n/a B 91% |

-
-
67-56-1
methanol

-
-
103-82-2
phenylacetic acid

-
A
-
142-08-5
2-hydroxypyridin

-
B
-
101-41-7
benzeneacetic acid methyl ester

| Conditions | Yield |
|---|---|
| With 2,2'-dipyridyl carbonate; dmap In dichloromethane for 1h; Ambient temperature; | A 90% B 89% |

-
-
103-82-2
phenylacetic acid

-
-
109-79-5
1-butanethiol

-
A
-
142-08-5
2-hydroxypyridin

-
B
-
54829-39-9
S-butyl 2-phenylethanethioate

| Conditions | Yield |
|---|---|
| With 2,2'-dipyridyl carbonate; dmap In dichloromethane for 1h; Ambient temperature; | A 90% B 82% |

-
-
56-41-7
L-alanin

-
-
89985-91-1
tert-butyl pyridin-2-yl carbonate

-
A
-
142-08-5
2-hydroxypyridin

-
B
-
15761-38-3
L-N-Boc-Ala

| Conditions | Yield |
|---|---|
| With triethylamine In water; N,N-dimethyl-formamide for 8h; Ambient temperature; | A n/a B 90% |

-
-
34780-32-0
N-cyclohexyl-N'-(t-butyl)thiourea

-
A
-
142-08-5
2-hydroxypyridin

-
B
-
1202-53-5
t-butylcyclohexylcarbodiimide

| Conditions | Yield |
|---|---|
| With di-2-pyridyl thionocarbonate; dmap In acetonitrile for 12h; Ambient temperature; | A n/a B 90% |
-
-
14327-04-9
N-tert-butyl-N'-phenylthiourea

-
A
-
142-08-5
2-hydroxypyridin

-
B
-
2219-34-3
N-((tert-butylimino)methylene)aniline

| Conditions | Yield |
|---|---|
| With di-2-pyridyl thionocarbonate; dmap In acetonitrile for 1h; Ambient temperature; | A n/a B 90% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-hydroxypyridin With palladium 10% on activated carbon; hydrogen; acetic acid at 24 - 28℃; for 15h; Stage #2: In methanol | 100% |
| With palladium on activated charcoal; hydrogen; acetic acid at 20℃; under 760.051 Torr; for 15h; Time; | 100% |
| With nickel-copper at 200 - 235℃; under 29420.3 Torr; Hydrogenation; |

| Conditions | Yield |
|---|---|
| With sodium hydride; lithium bromide In 1,2-dimethoxyethane; N,N-dimethyl-formamide | 100% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 20℃; for 5.5h; | 100% |

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 24h; | 100% |
| at 25 - 30℃; for 24h; |

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 24h; | 100% |
| at 25 - 30℃; |

| Conditions | Yield |
|---|---|
| In ethanol; water at 20℃; for 24h; | 100% |
-
-
142-08-5
2-hydroxypyridin

-
-
23329-69-3, 454677-87-3
cis-di-2-propanolato-bis(8-quinolinato)-titanium(IV)

-
-
1758-54-9, 79462-42-3, 81563-77-1
1-pyridin-2-yl-ethanone oxime

| Conditions | Yield |
|---|---|
| In toluene at 100 - 120℃; | 98.1% |


| Conditions | Yield |
|---|---|
| With caesium carbonate; copper(I) oxide; trans-N,N'-bis(pyridin-2-ylmethylene)cyclohexane-1,2-diamine In acetonitrile at 82℃; for 24h; Conversion of starting material; activated 3 A molecular sieve (KnNa12,-n[(AlO2)12(SiO2)12]); | 98% |
| With potassium phosphate; copper(l) iodide; N-(2-fluorophenyl)picolinamide at 150℃; for 24h; Green chemistry; | 98% |
| With copper(l) iodide; potassium carbonate; trans-N,N'-dimethylcyclohexane-1,2-diamine In toluene for 18h; Heating; | 84% |
| With copper(l) iodide; potassium carbonate In N,N-dimethyl-formamide at 150℃; for 6h; | 72% |

| Conditions | Yield |
|---|---|
| In benzene byproducts: CH4, Et2O; under N2; a soln. of a ligand in benzene was added to a benzene soln. ofGa-contg. compd. and stirred for 4 h; the solvent was removed under reduced pressure; elem. anal.; | 98% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide; tetraethoxy orthosilicate In o-xylene Heating; | 98% |

| Conditions | Yield |
|---|---|
| With propylamine; bis(1,5-cyclooctadiene)diiridium(I) dichloride; [O,O’-((S)-1,1´-dinaphthyl-2,2´-diyl)-N,N´-di-(S,S)-1-(2-methoxyphenyl)ethylphosphoramidite]; caesium carbonate In tetrahydrofuran at 50℃; for 1.5h; Schlenk technique; Inert atmosphere; enantioselective reaction; | 98% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 2h; | 98% |
-
-
142-08-5
2-hydroxypyridin

-
-
110-80-5
2-ethoxy-ethanol

-
-
23329-69-3, 454677-87-3
cis-di-2-propanolato-bis(8-quinolinato)-titanium(IV)

| Conditions | Yield |
|---|---|
| In toluene at 100 - 120℃; Reflux; | 97.9% |

-
-
142-08-5
2-hydroxypyridin

-
-
23329-69-3, 454677-87-3
cis-di-2-propanolato-bis(8-quinolinato)-titanium(IV)

-
-
5007-50-1
1-(2-furyl)ethanone oxime

| Conditions | Yield |
|---|---|
| In toluene at 100 - 120℃; | 97.8% |

-
-
142-08-5
2-hydroxypyridin

-
-
14049-60-6
tetra-n-butylammonium octabromodirhenate(III)

-
-
104647-37-2
Re2(2-hydroxypyridinate)4Br2

| Conditions | Yield |
|---|---|
| In pentan-1-ol the soln. of Re complex and ligand was refluxed for 15 h under N2; ppt. was filtered off, washed with n-pentanol, ether, dried in vac., elem. anal.; | 97% |
-
-
142-08-5
2-hydroxypyridin

-
-
313683-65-7
C5(CH3)5Fe(CO)Si(CH3)2Si(CH3)2OC5H4N

-
-
375388-38-8
η5-C5Me5(CO)Fe(H)-(SiMe2O(2-C5H4N))2

| Conditions | Yield |
|---|---|
| In toluene at room temp.; elem. anal.; | 97% |

| Conditions | Yield |
|---|---|
| In benzene byproducts: C2H6, Et2O; under N2; a soln. of a ligand in benzene was added to a benzene soln. ofGa-contg. compd. and stirred for 4 h; the solvent was removed under reduced pressure; elem. anal.; | 97% |

| Conditions | Yield |
|---|---|
| In benzene byproducts: C2H6, Et2O; under N2; a soln. of a ligand in benzene was added to a benzene soln. ofGa-contg. compd. and stirred for 4 h; the solvent was removed under reduced pressure; elem. anal.; | 97% |
2-Hydroxypyridine Consensus Reports
2-Hydroxypyridine Specification
The 2-Oxopyridine with CAS registry number of 142-08-5 is also called 2-Hydroxypyridine. The IUPAC name is 1H-pyridin-2-one. Its EINECS registry number is 205-520-3. In addition, the molecular formula is C5H5NO and the molecular weight is 95.10. It is a kind of white to light yellow crystal and belongs to the classes of Pyridines; Pyridine; Pyridines derivates; (intermediate of benzoxazole); Nucleotides and Nucleosides; Bases & Related Reagents; Nucleotides. And it should be stored in a cool and dry place.
Physical properties about this chemical are: (1)ACD/LogP: -0.58; (2)ACD/LogD (pH 5.5): -0.58; (3)ACD/LogD (pH 7.4): -0.58; (4)ACD/BCF (pH 5.5): 1; (5)ACD/BCF (pH 7.4): 1; (6)ACD/KOC (pH 5.5): 11.52; (7)ACD/KOC (pH 7.4): 11.52; (8)#H bond acceptors: 2; (9)#H bond donors: 1; (10)Polar Surface Area: 20.31 Å2; (11)Index of Refraction: 1.513; (12)Molar Refractivity: 25.74 cm3; (13)Molar Volume: 85.5 cm3; (14)Polarizability: 10.2 ×10-24cm3; (15)Surface Tension: 35.7 dyne/cm; (16)Density: 1.111 g/cm3; (17)Flash Point: 181.1 °C; (18)Enthalpy of Vaporization: 56.57 kJ/mol; (19)Boiling Point: 323.7 °C at 760 mmHg; (20)Vapour Pressure: 0.000257 mmHg at 25°C.
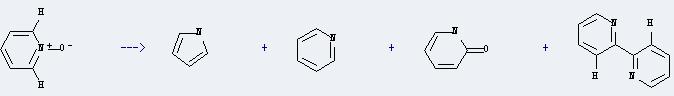
Preparation of 2-Oxopyridine: it can be prepared by pyridine 1-oxide. There are some other products, such as pyridin-2-yl-acetonitrile, [2,2']bipyridinyl, pyrrole and pyridine. The yield of this reaction is about 31% at reaction temperature of 750-850 °C.
Uses of 2-Oxopyridine: it can be used to get 2-chloro-pyridine. This reaction will need reagents N-chloruccinimide and PPh3 and solvent dioxane. The reaction time is 4 hours by heating . The yield is about 43%.

When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes, respiratory system and skin. There is limited evidence of a carcinogenic effect. During using it, wear suitable protective clothing, gloves and eye/face protection and do not breathe dust. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: c1cc[nH]c(=O)c1
(2)InChI: InChI=1/C5H5NO/c7-5-3-1-2-4-6-5/h1-4H,(H,6,7)
(3)InChIKey: UBQKCCHYAOITMY-UHFFFAOYAK
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 410mg/kg (410mg/kg) | Toxicon. Vol. 23, Pg. 815, 1985. | |
| mouse | LD50 | intravenous | 750mg/kg (750mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 93, Pg. 143, 1953. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View