-
Name
2-Methyl-6-nitroaniline
- EINECS 209-329-6
- CAS No. 570-24-1
- Article Data41
- CAS DataBase
- Density 1.269 g/cm3
- Solubility <0.1 g/100 mL at 23 °C in water
- Melting Point 93-96 °C(lit.)
- Formula C7H8N2O2
- Boiling Point 301.4 °C at 760 mmHg
- Molecular Weight 152.153
- Flash Point 136.1 °C
- Transport Information UN 2660 6.1/PG 3
- Appearance redish-brown solid
- Safety 28-36/37-45-61-28A
- Risk Codes 23/24/25-33-51/53
-
Molecular Structure
-
Hazard Symbols
 T,
T,  N,
N,  Xi
Xi
- Synonyms 1-Amino-2-methyl-6-nitrobenzene;6-Nitro-o-toluidine;2-Amino-3-nitrotoluene;Benzenamine, 2-methyl-6-nitro- (9CI);2-Methyl-6-nitro-benzenamine;Benzenamine, 2-methyl-6-nitro-;2-methyl-6-nitro-aniline;2-Methyl-6-Nitro-toluidine;2-Methyl-6-nitrobenzenamine;
- PSA 71.84000
- LogP 2.58980
Synthetic route

| Conditions | Yield |
|---|---|
| Stage #1: 2-aminotoluene-5-sulfonic acid With acetic acid; zinc(II) oxide at 80℃; for 4h; Stage #2: With nitric acid at 10 - 12℃; for 2h; Stage #3: With hydrogenchloride In water at 100℃; for 1h; | 82% |

| Conditions | Yield |
|---|---|
| With bromine; silver nitrate; triphenylphosphine In acetonitrile at 20℃; for 0.0833333h; | A 15% B 67% |
| With 4-nitro-4-methyl-2,3,5,6-tetrabromo-2,5-cyclohexadien-1-one In acetic acid for 3h; Ambient temperature; | A 30% B 40% |
| With acetic anhydride anschliessend mit wss.Salpetersaeure und Erwaermen des Reaktionsprodukts mit wss.Salzsaeure; |

| Conditions | Yield |
|---|---|
| Stage #1: 2-methyl-6-nitroazidobenzene With triphenylphosphine In diethyl ether at 20℃; for 1h; Stage #2: isobutyraldehyde In chloroform for 144h; aza-Wittig reaction; Heating; Stage #3: With carbon monoxide; 1,3-bis-(diphenylphosphino)propane; 1,10-phenanthroline hydrate; bis(dibenzylideneacetone)-palladium(0) In N,N-dimethyl-formamide at 70℃; under 4560.31 Torr; for 21h; Further stages.; | A 8% B 62% C 11% |

-
-
120-66-1
N-(2-methylphenyl)acetamide

-
A
-
570-24-1
2-Methyl-6-nitroaniline

-
B
-
99-52-5
2-methyl-4-nitro-benzenamine

| Conditions | Yield |
|---|---|
| Stage #1: N-(2-methylphenyl)acetamide With nitric acid at 10 - 12℃; Stage #2: With hydrogenchloride | A 60% B n/a |
| With nitric acid; acetic acid at 7 - 8℃; man laesst bei Zimmertemperatur stehen, giesst in wenig Wasser, filtriert, und erhitzt das Nitrierungsprodukt mit Salzsaeure; destilliert man hierauf mit Wasserdampf und erhaelt man mit Natronlauge die freie Base; | |
| With nitric acid Nitrierung und Verseifung; | |
| With ammonium nitrate; nitric acid; acetic acid Nitrierung und Verseifung; | |
| With water; nitric acid; acetic acid man versetzt den abfiltrierten Niederschlag mit konz.Salzsaeure und destilliert mit Wasserdampf, wobei 3-Nitro-2-amino-toluol uebergeht, waehrend 5-Nitro-2-amino-toluol in der salzsauren Loesung zurueckbleibt; |
-
-
59907-22-1
N-(2-methyl-6-nitrophenyl)acetamide

-
A
-
570-24-1
2-Methyl-6-nitroaniline

-
B
-
2719-15-5
2-methyl-4-nitroacetanilide

| Conditions | Yield |
|---|---|
| With hydrogenchloride | A 46% B n/a |

| Conditions | Yield |
|---|---|
| With acetic acid und Erwaermen des Reaktionsprodukts mit wss.Salzsaeure; |
-
-
120-66-1
N-(2-methylphenyl)acetamide

-
A
-
570-24-1
2-Methyl-6-nitroaniline

-
B
-
2719-15-5
2-methyl-4-nitroacetanilide

| Conditions | Yield |
|---|---|
| With nitric acid; acetic anhydride; acetic acid at 15 - 20℃; ueber mehrere Stufen; |

| Conditions | Yield |
|---|---|
| With ammonia at 150 - 160℃; unter Druck; |
-
-
59907-22-1
N-(2-methyl-6-nitrophenyl)acetamide

-
-
124-41-4
sodium methylate

-
-
570-24-1
2-Methyl-6-nitroaniline

| Conditions | Yield |
|---|---|
| Rate constant; |
-
-
59907-22-1
N-(2-methyl-6-nitrophenyl)acetamide

-
-
570-24-1
2-Methyl-6-nitroaniline

| Conditions | Yield |
|---|---|
| With sodium methylate | |
| With hydrogenchloride | |
| (hydrolysis); |
-
-
80-28-4
N-(2-methylphenyl)-p-toluenesulphonamide

-
A
-
570-24-1
2-Methyl-6-nitroaniline

-
B
-
7477-94-3
2-methyl-4,6-dinitroaniline

| Conditions | Yield |
|---|---|
| bei hoeherer Temperatur erhaelt man ein Gemisch von Nitrierungsprodukten.Hydrolysis; |

| Conditions | Yield |
|---|---|
| With sulfuric acid Schuetteln des Reaktionsprodukts mit Salpeterschwefelsaeure bei hoechstens 50grad und Erhitzen nach Zusatz von Wasser auf 140-150grad; |

| Conditions | Yield |
|---|---|
| With mineral acid |

-
-
570-24-1
2-Methyl-6-nitroaniline

| Conditions | Yield |
|---|---|
| durch Abspaltung der Sulfogruppe; |

| Conditions | Yield |
|---|---|
| (i) HNO3, (ii) aq. HCl; Multistep reaction; | |
| With nitric acid | |
| Multi-step reaction with 2 steps 1: nitric acid; acetic anhydride / dichloromethane 2: hydrogenchloride / water / Reflux View Scheme | |
| Stage #1: N-(2-methylphenyl)acetamide With nitric acid In acetic anhydride at 10 - 12℃; for 2.5h; Stage #2: With sulfuric acid In water for 3h; Reflux; | 39.78 g |
-
-
99-08-1
1-methyl-3-nitrobenzene

-
A
-
570-24-1
2-Methyl-6-nitroaniline

-
B
-
99-52-5
2-methyl-4-nitro-benzenamine

-
C
-
89-62-3
4-methyl-2-nitroaniline

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; N,N,N-trimethylhydrazinium iodide In dimethyl sulfoxide Ambient temperature; Yield given. Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| With nitric acid; acetic acid man kocht das entstehende Acetylderiv.in alkoh.Loesung mit der waessr.Kalilauge,wodurch nur das Acetylderiv.des 5-Nitro-2-amino-toluols verseift wird; das Acetylderiv.des 3-Nitro-2-amino-toluols verseift man mit konz.Salzsaeure; |

| Conditions | Yield |
|---|---|
| at 150 - 160℃; unter Druck; |

| Conditions | Yield |
|---|---|
| anschliessenden Schuetteln mit Salpeterschwefelsaeure unterhalb 50grad und Erhitzen mit Wasser auf 140-150grad; |
-
-
7647-01-0
hydrogenchloride

-
-
60-29-7
diethyl ether

-
A
-
570-24-1
2-Methyl-6-nitroaniline

-
B
-
99-52-5
2-methyl-4-nitro-benzenamine

| Conditions | Yield |
|---|---|
| at 0℃; |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: 12 - 13 °C 2.1: 65 percent HNO3 / 10 - 12 °C 2.2: 60 percent / conc. HCl View Scheme | |
| Multi-step reaction with 2 steps 2: HNO3 View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 60 percent / HNO3 2: concd. HCl / 7 h / Heating View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 2: acetic acid anhydride; glacial acetic acid; nitric acid / 15 - 20 °C / ueber mehrere Stufen View Scheme | |
| Multi-step reaction with 3 steps 1: triethylamine / dichloromethane 2: nitric acid; acetic anhydride / dichloromethane 3: hydrogenchloride / water / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: (nitration) 2: aq. HCl View Scheme |
-
-
570-24-1
2-Methyl-6-nitroaniline

-
-
532934-93-3
4-iodo-2-methyl-6-nitroaniline

| Conditions | Yield |
|---|---|
| With Iodine monochloride; acetic acid at 30℃; for 3h; | 100% |
| With sodium acetate; Iodine monochloride; acetic acid at 80℃; for 0.833333h; | 98% |
| With N-iodo-succinimide; acetic acid at 50 - 55℃; for 6h; Inert atmosphere; | 97% |
| With Iodine monochloride; acetic acid | |
| With Iodine monochloride; sodium hydrogencarbonate In methanol; dichloromethane at 20℃; for 6h; |

| Conditions | Yield |
|---|---|
| With hydrogen In ethyl acetate at 60℃; under 7500.75 Torr; for 20h; Autoclave; Green chemistry; | 99% |
| With hydrogen In ethyl acetate at 60℃; under 7500.75 Torr; for 20h; Autoclave; | 99% |
-
-
570-24-1
2-Methyl-6-nitroaniline

-
-
62790-50-5
4-chloro-2-methyl-6-nitrophenylamine

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide In methanol at 60℃; for 4h; | 98% |
| With N-chloro-succinimide; acetic acid | 92% |
| With N-chloro-succinimide; acetic acid at 50 - 55℃; for 6h; Inert atmosphere; | 92% |

| Conditions | Yield |
|---|---|
| With acetic acid; sodium nitrite for 0.333333h; | 98% |
| With acetic acid; sodium nitrite In water | 98% |
| With acetic acid; sodium nitrite In water at 20℃; for 0.333333h; Inert atmosphere; | 97% |
-
-
570-24-1
2-Methyl-6-nitroaniline

-
-
100-39-0
benzyl bromide

-
-
161958-74-3
1-Benzyloxy-4-methyl-2-phenyl-1H-benzoimidazole

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran for 16h; Heating; | 98% |
-
-
570-24-1
2-Methyl-6-nitroaniline

-
-
123-54-6
acetylacetone

-
-
864652-84-6
3-(2-mehtyl-6-nitrophenylhydrazono)pentane-2,4-dione

| Conditions | Yield |
|---|---|
| Stage #1: 2-Methyl-6-nitroaniline With hydrogenchloride; sodium nitrite Stage #2: acetylacetone With sodium hydroxide; sodium acetate In methanol; water at 0 - 20℃; for 1h; Further stages.; | 98% |
-
-
570-24-1
2-Methyl-6-nitroaniline

-
-
77811-44-0
4-bromo-2-methyl-6-nitroaniline

| Conditions | Yield |
|---|---|
| With hydrogen bromide; dihydrogen peroxide In ethanol; water at 60℃; for 1h; Product distribution / selectivity; | 97.7% |
| With N-Bromosuccinimide; acetic acid at 50 - 55℃; for 6h; Inert atmosphere; | 93% |
| With sodium tungstate; dihydrogen peroxide; acetic acid; potassium bromide for 1h; Ambient temperature; | 90% |
| With sodium tungstate; sodium perborate; sulfuric acid; acetic anhydride; potassium bromide In acetic acid for 1h; Ambient temperature; | 69% |
| With N-Bromosuccinimide In tetrachloromethane Substitution; bromination; | 60% |

| Conditions | Yield |
|---|---|
| With hydrogen; palladium 10% on activated carbon In ethanol at 20℃; under 760.051 Torr; for 4h; | 97% |
| With hydrogenchloride; tin for 1h; Reflux; | 85.8% |
| Stage #1: 2-Methyl-6-nitroaniline With tin(ll) chloride In ethyl acetate for 6h; Reflux; Stage #2: With water; sodium hydrogencarbonate In ethyl acetate Cooling; | 70% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In dichloromethane; water at 0℃; for 1h; | 97% |
| With sodium carbonate In water for 0.5h; pH=8 - 9; | |
| With sodium carbonate In water for 2h; |
-
-
570-24-1
2-Methyl-6-nitroaniline

-
-
79-10-7
acrylic acid

-
-
1296194-25-6
3-(2-methyl-6-nitrophenylamino)propionic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid at 25 - 80℃; Inert atmosphere; | 96% |
-
-
570-24-1
2-Methyl-6-nitroaniline

-
-
707-07-3
orthobenzoic acid trimethyl ester

-
-
3659-77-6
4-methyl-2-phenyl-1H-benzimidazole

| Conditions | Yield |
|---|---|
| With indium; acetic acid In ethyl acetate for 1h; Reflux; Inert atmosphere; | 96% |

| Conditions | Yield |
|---|---|
| With potassium phosphate; tris-(dibenzylideneacetone)dipalladium(0); C35H38NO4P In 1,4-dioxane at 100℃; for 18h; | 96% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-Methyl-6-nitroaniline With sodium nitrite In sulfuric acid; acetic acid Stage #2: With potassium iodide In sulfuric acid; water; acetic acid Further stages.; | 95% |
| Stage #1: 2-Methyl-6-nitroaniline With hydrogenchloride In water at 0℃; for 0.25h; Stage #2: With sodium nitrite In water at 0℃; for 1.67h; Stage #3: With potassium iodide In water at 0℃; for 2.17h; | 88% |
| Stage #1: 2-Methyl-6-nitroaniline With sulfuric acid; sodium nitrite In acetic acid at 15 - 75℃; Stage #2: With urea; potassium iodide | 79% |
-
-
570-24-1
2-Methyl-6-nitroaniline

-
-
1453211-57-8
2-chloro-5-[(3,5-dimethoxyphenyl)methoxy]pyrimidine

| Conditions | Yield |
|---|---|
| With caesium carbonate; bis(dibenzylideneacetone)-palladium(0); XPhos In N,N-dimethyl acetamide at 110℃; for 3h; Inert atmosphere; | 94% |
-
-
570-24-1
2-Methyl-6-nitroaniline

-
-
41085-43-2
2-bromo-3-nitrotoluene

| Conditions | Yield |
|---|---|
| With hydrogen bromide; copper(I) bromide; sodium nitrite In 1,4-dioxane; water at 0 - 60℃; | 93% |
| Stage #1: 2-Methyl-6-nitroaniline With hydrogen bromide In 1,4-dioxane; water at 60℃; for 0.75h; Inert atmosphere; Stage #2: With sodium nitrite In 1,4-dioxane; water at 0 - 8℃; for 2.33333h; Inert atmosphere; Stage #3: With hydrogen bromide; copper(I) bromide In 1,4-dioxane; water at 20 - 60℃; for 20.5h; Inert atmosphere; | 82% |
| Stage #1: 2-Methyl-6-nitroaniline With hydrogen bromide In 1,4-dioxane; water at 60℃; for 0.75h; Inert atmosphere; Stage #2: With sodium nitrite In 1,4-dioxane; water at 0 - 5℃; Inert atmosphere; Stage #3: With hydrogen bromide; copper(I) bromide In 1,4-dioxane; water at 24 - 60℃; Inert atmosphere; | 81% |
-
-
570-24-1
2-Methyl-6-nitroaniline

-
-
106-95-6
allyl bromide

-
-
161958-75-4
1-allyloxy-4-methyl-2-vinylbenzimidazole

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran for 16h; Heating; | 92% |
-
-
570-24-1
2-Methyl-6-nitroaniline

-
-
879-18-5
naphthalene-1-carbonic acid chloride

-
-
212503-73-6
Naphthalene-1-carboxylic acid (2-methyl-6-nitro-phenyl)-amide

| Conditions | Yield |
|---|---|
| With pyridine In tetrahydrofuran for 6h; Ambient temperature; | 91% |
-
-
570-24-1
2-Methyl-6-nitroaniline

-
-
108-24-7
acetic anhydride

-
-
59907-22-1
N-(2-methyl-6-nitrophenyl)acetamide

| Conditions | Yield |
|---|---|
| In chloroform for 12h; Time; Reflux; | 90.2% |
| With sulfuric acid |
-
-
570-24-1
2-Methyl-6-nitroaniline

-
-
286-20-4
cyclohexane-1,2-epoxide

-
-
6628-80-4
trans-2-chlorocyclohexanol

| Conditions | Yield |
|---|---|
| With bismuth(III) chloride In cyclohexane at 20℃; | A 90% B n/a |

-
-
570-24-1
2-Methyl-6-nitroaniline

-
-
1431381-87-1
1,3-bis(2-methyl-6-nitrophenyl)-2λ4-diazathia-1,2-diene

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane; disulfur dichloride In chloroform at -30℃; for 2h; Reflux; | 90% |

| Conditions | Yield |
|---|---|
| With sodium carbonate In water pH=9 - 10; | 89% |
-
-
570-24-1
2-Methyl-6-nitroaniline

-
-
431-03-8
dimethylglyoxal

-
-
17635-19-7
5-methyl-2,3-dimethylquinoxaline

| Conditions | Yield |
|---|---|
| With indium; indium(III) chloride In methanol for 2.5h; Reflux; Inert atmosphere; | 89% |

| Conditions | Yield |
|---|---|
| With indium; indium(III) chloride In methanol for 1.66667h; Reagent/catalyst; Reflux; Inert atmosphere; | 89% |
-
-
570-24-1
2-Methyl-6-nitroaniline

-
-
513-85-9
2.3-butanediol

-
-
17635-19-7
5-methyl-2,3-dimethylquinoxaline

| Conditions | Yield |
|---|---|
| With sodium hydroxide In toluene at 120℃; for 3h; Inert atmosphere; Sealed tube; Green chemistry; | 89% |
| With dodecacarbonyl-triangulo-triruthenium; cesiumhydroxide monohydrate; 1,3-bis-(diphenylphosphino)propane In tert-Amyl alcohol at 150℃; for 8h; Schlenk technique; Inert atmosphere; | 80% |

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran for 12h; Heating; | 87% |
-
-
570-24-1
2-Methyl-6-nitroaniline

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; acetic acid for 2h; Reflux; Inert atmosphere; | 87% |
| With N-Bromosuccinimide; acetic acid for 2h; Reflux; | 87% |

| Conditions | Yield |
|---|---|
| With sodium perborate; titanium(IV) hydroxide; acetic acid at 90 - 100℃; for 1h; Inert atmosphere; | 86.8% |
| With dihydrogen peroxide In acetic acid at 90℃; for 3h; | 48% |
| With sulfuric acid Diazotization.Ueberfuehrung des Diazoniumsulfats in das Nitrit und Behandeln mit Kupfer(I)-oxyd; |

| Conditions | Yield |
|---|---|
| With potassium iodide; sulfuric acid; sodium nitrite In ice-water; ethanol; dichloromethane | 84% |
-
-
570-24-1
2-Methyl-6-nitroaniline

-
-
84-11-7
9,10-phenanthrenequinone

-
-
112657-94-0
10-methyldibenzo[a,c]phenazine

| Conditions | Yield |
|---|---|
| With indium; acetic acid In methanol for 0.166667h; Reflux; Inert atmosphere; | 84% |
-
-
570-24-1
2-Methyl-6-nitroaniline

-
-
108-24-7
acetic anhydride

-
-
82344-53-4
N,N-diacetyl-6-nitro-o-toluidine

| Conditions | Yield |
|---|---|
| at 120℃; for 12.5h; | 83.7% |
2-Methyl-6-nitroaniline Chemical Properties
The molecular structure of 2-Methyl-6-nitroaniline (CAS NO.570-24-1):
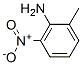
IUPAC Name: 2-Methyl-6-nitroaniline
Molecular Weight: 152.15062 g/mol
Molecular Formula: C7H8N2O2
Density: 1.269 g/cm3
Melting Point: 93-96 °C(lit.)
Boiling Point: 301.4 °C at 760 mmHg
Flash Point: 136.1 °C
Index of Refraction: 1.615
Molar Refractivity: 41.85 cm3
Molar Volume: 119.8 cm3
Surface Tension: 54.9 dyne/cm
Enthalpy of Vaporization: 54.16 kJ/mol
Vapour Pressure: 0.00105 mmHg at 25 °C
Water Solubility: <0.1 g/100 mL at 23 °C
XLogP3-AA: 2
H-Bond Donor: 1
H-Bond Acceptor: 3
Exact Mass: 152.058578
MonoIsotopic Mass: 152.058578
Topological Polar Surface Area: 69.2
Heavy Atom Count: 11
Canonical SMILES: CC1=C(C(=CC=C1)[N+](=O)[O-])N
InChI: InChI=1S/C7H8N2O2/c1-5-3-2-4-6(7(5)8)9(10)11/h2-4H,8H2,1H3
InChIKey: FCMRHMPITHLLLA-UHFFFAOYSA-N
EINECS: 209-329-6
Product Categories: Intermediates of Dyes and Pigments; Anilines, Aromatic Amines and Nitro Compounds; Amines; Phenyls & Phenyl-Het; Benzene derivates; API intermediates; Aromatics Compounds; Aromatics; Phenyls & Phenyl-Het
2-Methyl-6-nitroaniline Uses
2-Methyl-6-nitroaniline (CAS NO.570-24-1) is used in organic synthesis.
2-Methyl-6-nitroaniline Safety Profile
Hazard Codes:  T,
T,  N,
N,  Xi
Xi
Risk Statements: 23/24/25-33-51/53
R23/24/25:Toxic by inhalation, in contact with skin and if swallowed.
R33:Danger of cumulative effects.
R51/53:Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment.
Safety Statements: 28-36/37-45-61-28A
S28:After contact with skin, wash immediately with plenty of soap-suds.
S36/37:Wear suitable protective clothing and gloves.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S61:Avoid release to the environment. Refer to special instructions / safety data sheets.
RIDADR: UN 2660 6.1/PG 3
WGK Germany: 3
F: 10
Hazard Note: Toxic
HazardClass: IRRITANT
HS Code: 29214300
2-Methyl-6-nitroaniline Specification
2-Methyl-6-nitroaniline (CAS NO.570-24-1) is also named as 1-Amino-2-methyl-6-nitrobenzene ; 2-Methyl-6-nitro-benzenamine ; 6-Nitro-o-toluidine ; Benzenamine, 2-methyl-6-nitro- ; NSC 286 . 2-Methyl-6-nitroaniline (CAS NO.570-24-1) is redish-brown solid. It is insoluble in water. 2-Methyl-6-nitroaniline is sensitive to prolonged exposure to air. It is incompatible with acids, acid chlorides, acid anhydrides, chloroformates, and strong oxidizers.Flash point data for 2-Methyl-6-nitroaniline are not available, but it is probably combustible.
Related Products
- 2-Methyl-6-nitroaniline
- 57026-80-9
- 5702-75-0
- 57028-96-3
- 5703-26-4
- 57-03-4
- 570-36-5
- 570391-19-4
- 570394-17-1
- 5704-03-0
- 5704-04-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View