-
Name
2-Methyltetrahydrofuran
- EINECS 202-507-4
- CAS No. 96-47-9
- Article Data249
- CAS DataBase
- Density 0.863 g/cm3
- Solubility water: 15 g/100 mL (25 °C)
- Melting Point -136 °C
- Formula C5H10O
- Boiling Point 79.9 °C at 760 mmHg
- Molecular Weight 86.1338
- Flash Point 10.4°F
- Transport Information UN 2536 3/PG 2
- Appearance Clear,colorless liquid
- Safety 16-23-39-33-26
- Risk Codes 11-19-36/37
-
Molecular Structure
-
Hazard Symbols
 F,
F, Xi
Xi
- Synonyms Furan, 2-methyl-tetrahydro-;(2R)-2-methyloxolane;2-Methyloxolane;Tetrahydrosylvan;(2S)-2-methyloxolane;74069-67-3;Furan, tetrahydro-2-methyl-;Tetrahydro-2-methyl-furan;
- PSA 9.23000
- LogP 1.18530
Synthetic route

| Conditions | Yield |
|---|---|
| With 3% Pd/C; hydrogen In isopropyl alcohol at 219.84℃; under 25858.1 Torr; for 5h; Inert atmosphere; | 100% |
| With palladium-aluminum at 150℃; Hydrogenation; | |
| With osmium at 80℃; Hydrogenation; |

| Conditions | Yield |
|---|---|
| With hydrogen In isopropyl alcohol under 30003 Torr; for 5h; Reagent/catalyst; Autoclave; | 99% |
| Trichlorbutylstannan for 0.24h; | 96% |
| Nafion-H at 135℃; for 5h; | 90% |

| Conditions | Yield |
|---|---|
| With hydrogen In hexane at 180℃; for 4h; Temperature; Reagent/catalyst; Solvent; Autoclave; | 98% |
| Multi-step reaction with 2 steps 1: hydrogen / 1,4-dioxane / 2 h / 190 °C / 30003 Torr / Autoclave 2: hydrogen / 1,4-dioxane / 5 h / 190 °C / 30003 Torr / Autoclave View Scheme | |
| Multi-step reaction with 2 steps 1: hydrogen / 1,4-dioxane / 2 h / 190 °C / 30003 Torr / Autoclave 2: hydrogen / 1,4-dioxane / 5 h / 190 °C / 30003 Torr / Autoclave View Scheme |

| Conditions | Yield |
|---|---|
| With palladium on silica; copper phyllosilicate; hydrogen at 180℃; Reagent/catalyst; Inert atmosphere; | 97.1% |
| Stage #1: furfural With hydrogen at 200℃; under 760.051 Torr; Flow reactor; Stage #2: With hydrogen at 120℃; under 760.051 Torr; Temperature; Flow reactor; | 96.3% |
| With hydrogen; 5% palladium over charcoal; copper chromite catalyst E 403-TU at 175℃; under 760.051 Torr; Product distribution / selectivity; In the presence of quartz glass rings (as an evaporation zone); |

| Conditions | Yield |
|---|---|
| With tris(2,4-pentanedionato)ruthenium(III); N-butyl-N'-(4-sulfobutyl)-imidazolium p-toluenesulfonate; hydrogen; [2-((diphenylphospino)methyl)-2-methyl-1,3-propanediyl]bis[diphenylphosphine] at 160℃; under 75007.5 Torr; for 18h; Inert atmosphere; Autoclave; Ionic liquid; | 95% |
| Multi-step reaction with 2 steps 1: sodium; alcohol 2: hydrochloric acid; alcohol / 150 °C / im Druckrohr View Scheme | |
| Multi-step reaction with 2 steps 1: sodium; xylene 2: HI / auf dem Wasserbad und folgenden Reduzieren mit Zinkstaub und Eisessig View Scheme |

| Conditions | Yield |
|---|---|
| With bromopentacarbonylmanganese(I); phenylsilane In toluene at 100℃; for 24h; Catalytic behavior; Time; Temperature; Reagent/catalyst; Inert atmosphere; Schlenk technique; | 95% |
| With Cu/SiO2 at 220℃; under 22502.3 Torr; Temperature; | 93% |
| With ammonium hexafluorophosphate; Λ(+)-tris(pentane-2,5-dionato)ruthenium; N-butyl-N'-(4-sulfobutyl)-imidazolium p-toluenesulfonate; hydrogen; [2-((diphenylphospino)methyl)-2-methyl-1,3-propanediyl]bis[diphenylphosphine] at 160℃; under 75007.5 Torr; for 18h; Inert atmosphere; | 92% |

| Conditions | Yield |
|---|---|
| With N-iodo-succinimide In chlorobenzene for 2h; Irradiation; | A 94% B 1% C n/a |
| With N-iodo-succinimide In chlorobenzene for 2h; Product distribution; Irradiation; var. irradiat. times, temps. and light cond.; | A 94% B 1% C n/a |

-
-
41968-76-7
(C4H9)3SnOCH(CH3)CH2CH2CH2Cl

-
A
-
96-47-9
2-methyltetrahydrofuran

-
B
-
1461-22-9
tributyltin chloride

| Conditions | Yield |
|---|---|
| decompn. of the crude compound at 120°C (0.5 h); | A 93% B n/a |
| decompn. of the crude compound at 120°C (0.5 h); | A 93% B n/a |

| Conditions | Yield |
|---|---|
| With Nd magnetically supported catalyst In dichloromethane at 83℃; for 24h; Schlenk technique; Inert atmosphere; | 91% |
| With aluminium(III) triflate In nitromethane at 101℃; for 1h; | 87% |
| With ytterbium(III) triflate at 120℃; Inert atmosphere; Ionic liquid; | 78% |

| Conditions | Yield |
|---|---|
| With hydrogen In para-xylene at 180℃; under 30003 Torr; for 48h; Glovebox; Sealed tube; chemoselective reaction; | 90% |

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene; iodine Irradiation; | 89% |
-
-
98-01-1
furfural

-
A
-
96-47-9
2-methyltetrahydrofuran

-
B
-
534-22-5
2-methylfuran

-
C
-
6032-29-7
(+/-)-2-pentanol

-
D
-
107-87-9
2-Pentanone

| Conditions | Yield |
|---|---|
| With hydrogen; Cu-based catalyst at 212℃; Product distribution; Further Variations:; Temperatures; reaction in vapour phase, fixed bed reactor; | A 3.3% B 88.6% C 4.8% D 2.7% |


| Conditions | Yield |
|---|---|
| With 30% Cu/SiO2 In methanol at 190℃; under 60006 Torr; for 10h; Reagent/catalyst; Solvent; Temperature; Autoclave; | A 16.3% B 82.9% |

| Conditions | Yield |
|---|---|
| With carbon dioxide; 5% Pd(II)/C(eggshell); hydrogen at 300℃; under 112511 Torr; Supercritical conditions; | A 82% B 6% |
-
-
114720-43-3
N-(4-Pentenyl-1-oxy)pyridine-2(1H)-thione

-
-
96-47-9
2-methyltetrahydrofuran

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile); tri-n-butyl-tin hydride In benzene at 80℃; for 0.333333h; Product distribution; Mechanism; | 80% |

| Conditions | Yield |
|---|---|
| With hydrogen In water at 130℃; for 4h; Reagent/catalyst; Solvent; Autoclave; | A 17% B 80% |
| With hydrogen In water at 150℃; under 37503.8 Torr; for 4h; Autoclave; | A 20% B 79% |
| With hydrogen In water at 130℃; for 24h; Reagent/catalyst; Autoclave; | A 77% B 12% |

| Conditions | Yield |
|---|---|
| With triethylsilane; trifluorormethanesulfonic acid In dichloromethane-d2 at 20℃; for 24h; NMR tube; | 80% |
| With hydrogen In neat (no solvent) at 90℃; under 15001.5 Torr; for 5h; | > 99 %Chromat. |

| Conditions | Yield |
|---|---|
| Stage #1: furfural With hydrogen at 200℃; under 760.051 Torr; Flow reactor; Stage #2: With hydrogen at 100℃; under 760.051 Torr; Temperature; Flow reactor; | A 79.3% B 20% |
| With hydrogen In isopropyl alcohol at 229.84℃; under 30003 Torr; for 2h; | A 10% B 57% |
| With carbon dioxide; palladium on activated charcoal; hydrogen In water at 80℃; for 0.333333h; |

| Conditions | Yield |
|---|---|
| Stage #1: furfural With hydrogen at 160℃; under 760.051 Torr; Flow reactor; Stage #2: With hydrogen at 130℃; under 760.051 Torr; Flow reactor; | A 13.7% B 78.2% |
| With hydrogen In 1,4-dioxane; isopropyl alcohol at 200℃; under 48754.9 Torr; for 6h; Reagent/catalyst; | A 36% B 45% |
| Multi-step reaction with 2 steps 1: isopropyl alcohol; 10% Pd/C / 7.5 h / 150 °C / Inert atmosphere 2: isopropyl alcohol; 10% Pd/C / 7.5 h / 180 °C / Inert atmosphere View Scheme |
-
-
3128-06-1
5-ketohexanoic acid

-
A
-
96-47-9
2-methyltetrahydrofuran

-
B
-
108-29-2
5-methyl-dihydro-furan-2-one

| Conditions | Yield |
|---|---|
| With hydrogen In isopropyl alcohol at 250℃; under 52505.3 Torr; for 24h; Temperature; Reagent/catalyst; | A 75% B 8% |
| With hydrogen In isopropyl alcohol at 250℃; under 52505.3 Torr; for 5h; Temperature; Reagent/catalyst; | A 22.7% B 64.5% |
-
-
111341-49-2
phenylthio-4-pentenol

-
A
-
96-47-9
2-methyltetrahydrofuran

-
B
-
821-09-0
n-Pent-4-enyl alcohol

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile); tri-n-butyl-tin hydride In various solvent(s) at 80℃; for 0.5h; Rate constant; Mechanism; Bu3Sn. effect in SH2 displacement of alkoxy radicals from sulphur, exo cyclization; | A 73% B 26% |
-
-
98-01-1
furfural

-
A
-
96-47-9
2-methyltetrahydrofuran

-
B
-
97-99-4
Tetrahydrofurfuryl alcohol

-
C
-
98-00-0
(2-furyl)methyl alcohol

| Conditions | Yield |
|---|---|
| Stage #1: furfural With hydrogen at 160℃; under 760.051 Torr; Flow reactor; Stage #2: With hydrogen at 120℃; under 760.051 Torr; Flow reactor; | A 13.1% B 70% C 9.1% |
| With Ni3Sn2; hydrogen In isopropyl alcohol at 20 - 179.84℃; for 12h; Autoclave; | A 5 %Chromat. B 5 %Chromat. C 91 %Chromat. |
| With hydrogen In isopropyl alcohol at 50℃; under 3750.38 Torr; Autoclave; |

-
-
123-76-2
levulinic acid

-
A
-
96-47-9
2-methyltetrahydrofuran

-
B
-
71-41-0
pentan-1-ol

-
C
-
108-29-2
5-methyl-dihydro-furan-2-one

| Conditions | Yield |
|---|---|
| With hydrogen In water at 150℃; under 37503.8 Torr; for 4h; Autoclave; | A 68% B 7% C 11% |
| With hydrogen; CuO0.95Co0.05/SiO2 In 1,4-dioxane at 265℃; under 18751.9 Torr; | |
| With hydrogen; CuO(80)SiO2(20) In 1,4-dioxane at 265℃; under 19135 Torr; Product distribution / selectivity; Inert atmosphere; |

-
-
123-76-2
levulinic acid

-
A
-
96-47-9
2-methyltetrahydrofuran

-
B
-
626-95-9
1,4-Pentanediol

-
C
-
6032-29-7
(+/-)-2-pentanol

-
D
-
108-29-2
5-methyl-dihydro-furan-2-one

| Conditions | Yield |
|---|---|
| With hydrogen In water at 150℃; for 4h; Reagent/catalyst; Autoclave; | A 67% B n/a C 9% D 14% |


| Conditions | Yield |
|---|---|
| With hydrogen In water at 150℃; under 37503.8 Torr; for 4h; Autoclave; | A 64% B 6% |
| With hydrogen In 1,4-dioxane at 330℃; Product distribution / selectivity; | |
| With hydrogen In 1,4-dioxane at 265℃; under 15001.5 Torr; Product distribution / selectivity; | |
| With hydrogen; CuO(80)SiO2(20) In 1,4-dioxane at 290℃; under 19135 Torr; Product distribution / selectivity; Inert atmosphere; | |
| With hydrogen; CuO(80)SiO2(20) In 1,4-dioxane at 265℃; under 15001.5 Torr; Product distribution / selectivity; Inert atmosphere; |
-
-
123-76-2
levulinic acid

-
A
-
96-47-9
2-methyltetrahydrofuran

-
B
-
6032-29-7
(+/-)-2-pentanol

-
C
-
71-41-0
pentan-1-ol

| Conditions | Yield |
|---|---|
| With hydrogen In water at 150℃; under 37503.8 Torr; for 4h; Autoclave; | A 63% B 18% C 10% |


| Conditions | Yield |
|---|---|
| With hydrogen In water at 150℃; under 37503.8 Torr; for 4h; Autoclave; | A 60% B 18% |
| Multi-step reaction with 2 steps 1: ruthenium-carbon composite; hydrogen / 1,4-dioxane / 5 h / 30003 Torr / Autoclave 2: ruthenium-carbon composite; hydrogen / isopropyl alcohol / 5 h / 30003 Torr / Autoclave View Scheme | |
| Multi-step reaction with 2 steps 1: hydrogen / water / 100 °C / 30003 Torr / Flow reactor; Green chemistry 2: hydrogen / water / 70 °C / 30003 Torr / Flow reactor; Green chemistry View Scheme |
-
-
123-76-2
levulinic acid

-
A
-
96-47-9
2-methyltetrahydrofuran

-
B
-
626-95-9
1,4-Pentanediol

-
C
-
6032-29-7
(+/-)-2-pentanol

-
D
-
71-41-0
pentan-1-ol

| Conditions | Yield |
|---|---|
| With hydrogen In water at 150℃; under 37503.8 Torr; for 4h; Autoclave; | A 60% B 10% C 23% D 6% |
| With hydrogen In water at 150℃; under 37503.8 Torr; for 4h; Autoclave; | A 25% B 27% C 34% D 7% |
| With hydrogen In water at 150℃; under 37503.8 Torr; for 4h; Autoclave; |

-
-
98-00-0
(2-furyl)methyl alcohol

-
A
-
96-47-9
2-methyltetrahydrofuran

-
B
-
97-99-4
Tetrahydrofurfuryl alcohol

-
C
-
534-22-5
2-methylfuran

| Conditions | Yield |
|---|---|
| With hydrogen In methanol at 25℃; for 1h; Reagent/catalyst; Sealed tube; Green chemistry; | A 8% B 52% C 25% |
| With Ni/Al2O3; hydrogen at 180℃; under 7500.75 Torr; Reagent/catalyst; Flow reactor; |

-
-
98-00-0
(2-furyl)methyl alcohol

-
A
-
96-47-9
2-methyltetrahydrofuran

-
B
-
97-99-4
Tetrahydrofurfuryl alcohol

| Conditions | Yield |
|---|---|
| With hydrogen In methanol at 25℃; under 750.075 Torr; for 1h; Reagent/catalyst; Sealed tube; Green chemistry; | A 25% B 52% |
| With water; hydrogen at 199.84℃; under 60006 Torr; for 3h; Autoclave; | |
| With hydrogen In ethanol at 60℃; under 2250.23 Torr; for 2h; | |
| With hydrogen at 200℃; under 760.051 Torr; Flow reactor; | A 5.3 %Chromat. B 94.5 %Chromat. |
-
-
96-47-9
2-methyltetrahydrofuran

-
-
90397-87-8
rac-5-iodopentan-2-ol

| Conditions | Yield |
|---|---|
| With aluminium trichloride; sodium iodide In acetonitrile at 0℃; for 2h; regioselective ring opening of var. 2 subst. tetrahydrofurans; | 100% |
| With aluminium trichloride; sodium iodide In acetonitrile at 0℃; for 2h; | 100% |
| With aluminum (III) chloride; sodium iodide In acetonitrile at 0 - 20℃; for 5h; Inert atmosphere; Schlenk technique; | 58% |
-
-
96-47-9
2-methyltetrahydrofuran

-
-
62-53-3
aniline

-
-
33342-99-3, 160751-58-6, 160751-59-7
(+/-)-2-methyl-1-phenylpyrrolidine

| Conditions | Yield |
|---|---|
| With carbon dioxide; alumina In toluene at 237℃; under 108011 Torr; Flow reactor; Supercritical conditions; | 100% |
| Stage #1: aniline With 1,8-diazabicyclo[5.4.0]undec-7-ene; trichlorophosphate In 5,5-dimethyl-1,3-cyclohexadiene at 90℃; for 0.25h; Stage #2: 2-methyltetrahydrofuran In 5,5-dimethyl-1,3-cyclohexadiene at 110℃; for 15h; Sealed tube; | 93% |
| With trimethylaluminum In toluene at 20 - 110℃; for 16h; | 78% |
-
-
96-47-9
2-methyltetrahydrofuran

-
-
1114543-34-8
1,5,9-trimesityldipyrromethene

-
-
1191-47-5
dibutylmagnesium

| Conditions | Yield |
|---|---|
| Stage #1: 1,5,9-trimesityldipyrromethene; dibutylmagnesium In toluene for 0.25h; Inert atmosphere; Schlenk technique; Glovebox; Stage #2: 2-methyltetrahydrofuran In toluene Inert atmosphere; Schlenk technique; Glovebox; | 100% |


| Conditions | Yield |
|---|---|
| With [(IPr=S)BiCl3]*CHCl3 In dichloromethane at 20℃; for 2h; Reagent/catalyst; regioselective reaction; | 98% |
| K[Pt(C2H4)Cl3] In 2-methyltetrahydrofuran for 24h; Ambient temperature; | 70% |
| With zinc |
-
-
96-47-9
2-methyltetrahydrofuran

-
-
55930-45-5
1,4-Diiodopentane

| Conditions | Yield |
|---|---|
| With O-phenyl phosphorodichloridate; sodium iodide for 24h; Heating; | 98% |
| With N,N-diethyl-1,1,1-trimethylsilanamine; methyl iodide In toluene at 80 - 90℃; for 103h; Ring cleavage; iodination; diiodation; diiodination; | 51% |
-
-
96-47-9
2-methyltetrahydrofuran

-
-
61915-52-4
2,2-Dimethylpropanoyl iodide

-
-
82131-08-6
4-pivaloyloxy-1-iodopentane

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; Green chemistry; | 98% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; Green chemistry; | 97% |

| Conditions | Yield |
|---|---|
| With molybdenum(V) chloride In 1,2-dichloro-ethane at 50℃; for 3h; | 96% |
| With zinc(II) oxide In neat (no solvent) at 0 - 20℃; Green chemistry; | 94% |
| With iron In 1,2-dichloro-ethane at 70℃; for 8h; Time; Schlenk technique; Inert atmosphere; regioselective reaction; | 91% |
-
-
96-47-9
2-methyltetrahydrofuran

-
-
36394-75-9
(S)-2-acetoxypropanoyl chloride

| Conditions | Yield |
|---|---|
| With bismuth(III) chloride In dichloromethane at 20℃; | 96% |
-
-
96-47-9
2-methyltetrahydrofuran

-
-
108-24-7
acetic anhydride

-
-
32864-71-4, 140238-21-7, 140238-22-8
1,4-diacetoxypentane

| Conditions | Yield |
|---|---|
| With aminosulfonic acid In acetic acid at 60℃; for 4h; | 95% |
| With sulfuric acid at 20℃; for 20h; | 70% |
| With ytterbium(III) triflate Heating; | 47% |
| at 170 - 180℃; | |
| at 240℃; |
-
-
96-47-9
2-methyltetrahydrofuran

-
-
100-07-2
4-methoxy-benzoyl chloride

-
-
27749-09-3
4-chloropentyl 4-methoxybenzoate

| Conditions | Yield |
|---|---|
| With zinc(II) oxide In neat (no solvent) at 0 - 20℃; for 2.5h; Green chemistry; regioselective reaction; | 95% |
| With iron In 1,2-dichloro-ethane at 70℃; Schlenk technique; Inert atmosphere; regioselective reaction; | 93% |
| With iron pentacarbonyl Heating; |

| Conditions | Yield |
|---|---|
| With bismuth(III) bromide In dichloromethane at 20℃; for 4h; | 95% |
| With palladium diacetate at 100℃; for 2h; Microwave irradiation; | 81.9% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; Green chemistry; | 95% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; Green chemistry; | 95% |
-
-
96-47-9
2-methyltetrahydrofuran

-
-
598-40-3
ethyl iodoacetate

-
-
149741-97-9
Propionic acid 4-iodo-1-methyl-butyl ester

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; Green chemistry; | 95% |
-
-
96-47-9
2-methyltetrahydrofuran

-
-
506-96-7
Acetyl bromide

-
A
-
26923-92-2
1-acetoxy-4-bromopentane

-
B
-
26923-93-3
acetic acid-(4-bromo-1-methyl-butyl ester)

| Conditions | Yield |
|---|---|
| With zinc(II) chloride 1.) 21 deg C, 0.5 h, 2.) reflux, 2.5 h; Yields of byproduct given. Title compound not separated from byproducts; | A 93% B n/a |
| With zinc(II) chloride 1.) 21 deg C, 0.5 h, 2.) reflux, 2.5 h; Yield given. Title compound not separated from byproducts; | A 93% B n/a |


| Conditions | Yield |
|---|---|
| Stage #1: 1-amino-3-methylbenzene With 1,8-diazabicyclo[5.4.0]undec-7-ene; trichlorophosphate at 90℃; for 0.25h; Stage #2: 2-methyltetrahydrofuran at 110℃; for 10h; Sealed tube; | 93% |

| Conditions | Yield |
|---|---|
| With bismuth(III) chloride In dichloromethane at 20℃; | 92% |

| Conditions | Yield |
|---|---|
| With iron In 1,2-dichloro-ethane at 70℃; Schlenk technique; Inert atmosphere; regioselective reaction; | 92% |

| Conditions | Yield |
|---|---|
| With zinc(II) oxide In neat (no solvent) at 0 - 20℃; for 2h; Green chemistry; regioselective reaction; | 92% |
| With iron In 1,2-dichloro-ethane at 70℃; Schlenk technique; Inert atmosphere; regioselective reaction; | 88% |
-
-
96-47-9
2-methyltetrahydrofuran

| Conditions | Yield |
|---|---|
| at -30 - 20℃; for 0.333333h; Darkness; | 92% |

-
-
96-47-9
2-methyltetrahydrofuran

-
-
13342-22-8
3,5-dimethyl-N-ethylaniline

| Conditions | Yield |
|---|---|
| With titanium tetrachloride; 1,8-diazabicyclo[5.4.0]undec-7-ene In dichloromethane; o-xylene at 120℃; for 14h; Sealed tube; | 92% |
2-Methyltetrahydrofuran Consensus Reports
Reported in EPA TSCA Inventory.
2-Methyltetrahydrofuran Specification
1. Introduction of 2-Methyltetrahydrofuran
2-Methyltetrahydrofuran, can also be called Tetrahydro-2-methylfuran; Furan, tetrahydro-2-methyl-; 2-Methyl-4,5-Dihydrofuran; 2-Methyloxolane; alpha-Methyltetrahydrofuran. The IUPAC Name of it is 2-Methyloxolane. It is stable, but highly flammable. Incompatible with oxidizing agents, strong acids, strong bases. May form explosive peroxides in storage, so often supplied with an inhibitor added. Keep away from heat, sparks, and flame. Keep away from sources of ignition. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Flammables-area.
2. Properties of 2-Methyltetrahydrofuran
Density: 0.86 g/cm3
Melting Point: -136 ºC
Boiling Point: 78-80 ºC
Refractive Index: 1.4046-1.4066
Flash Point: -11 ºC
Water Solubility: 15 g/100 mL (25 C)
Storage Temp.: Flammables area
H bond acceptors: 1
H bond donors: 0
Freely Rotating Bonds: 0
Polar Surface Area: 9.23 Å2
Index of Refraction: 1.41
Molar Refractivity: 24.76 cm3
Molar Volume: 99.7 cm3
Surface Tension: 25.4 dyne/cm
Enthalpy of Vaporization: 30.74 kJ/mol
Vapour Pressure: 96.8 mmHg at 25°C
Appearance: Clear,colorless liquid
Classification Code: Skin/Eye Irritant
3. Structure Descriptors of 2-Methyltetrahydrofuran
InChI: InChI=1/C5H10O/c1-5-3-2-4-6-5/h5H,2-4H2,1H3
InChIKey: JWUJQDFVADABEY-UHFFFAOYAM
Std. InChI: InChI=1S/C5H10O/c1-5-3-2-4-6-5/h5H,2-4H2,1H3
Std. InChIKey: JWUJQDFVADABEY-UHFFFAOYSA-N
4. Toxicity of 2-Methyltetrahydrofuran
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rabbit | LD50 | skin | 4500mg/kg (4500mg/kg) | "Toxicology of Drugs and Chemicals," Deichmann, W.B., New York, Academic Press, Inc., 1969Vol. -, Pg. 395, 1969. | |
| rat | LC50 | inhalation | 6000ppm/4H (6000ppm) | "Toxicology of Drugs and Chemicals," Deichmann, W.B., New York, Academic Press, Inc., 1969Vol. -, Pg. 395, 1969. |
5. Safety Information of 2-Methyltetrahydrofuran
Hazard Codes:
 F,
F, Xi
XiRisk Statements:11-19-36/37
R11:Highly flammable.
R19:May form explosive peroxides.
R36/37:Irritating to eyes and respiratory system.
Safety Statements:16-23-39-33-26
S16:Keep away from sources of ignition.
S23:Do not breathe vapour.
S39:Wear eye / face protection.
S33:Take precautionary measures against static discharges.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
RIDADR:UN 2536 3/PG 2
WGK Germany:2
RTECS:LU2800000
HazardClass:3
PackingGroup:II
HS Code:29321900
6. Preparation of 2-Methyltetrahydrofuran
2-Methyltetrahydrofuran can also be produced starting from levulinic acid. Cyclization and reduction gives γ-valerolactone
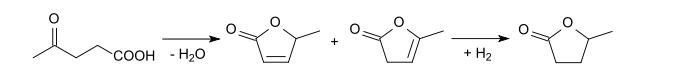
This lactone can be hydrogenated to 1,4-pentanediol, which can then be dehydrated to give 2-methyltetrahydrofuran
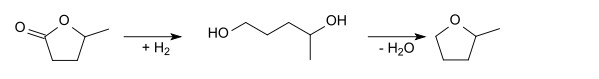
7. Use of 2-Methyltetrahydrofuran
2-Methyltetrahydrofuran is mainly used as solvents for resin, rubber, ethyl cellulose.And it also can be used as for the synthesis of anti-disease drugs such as Primaquine Phosphate in the pharmaceutical industry.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View