-
Name
3,4,5-Trimethoxyphenylacetonitrile
- EINECS 236-388-5
- CAS No. 13338-63-1
- Article Data36
- CAS DataBase
- Density 1.104 g/cm3
- Solubility Insoluble in water
- Melting Point 76-79 ºC
- Formula C11H13 N O3
- Boiling Point 327.9 °C at 760 mmHg
- Molecular Weight 207.229
- Flash Point 132.8 °C
- Transport Information
- Appearance white crystalline powder
- Safety S24/25
- Risk Codes R20/21/22
-
Molecular Structure
-
Hazard Symbols

- Synonyms Acetonitrile,(3,4,5-trimethoxyphenyl)- (6CI,7CI,8CI); (3,4,5-Trimethoxyphenyl)acetonitrile;2-(3,4,5-Trimethoxyphenyl)acetonitrile; 3,4,5-Trimethoxybenzeneacetonitrile;3,4,5-Trimethoxybenzyl cyanide; 3,4,5-Trimethoxybenzylnitrile; NSC 97556
- PSA 51.48000
- LogP 1.77848
Synthetic route
-
-
139109-56-1
Oxime of 3,4,5-trimethoxyphenylpyruvic acid

-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

| Conditions | Yield |
|---|---|
| With 1,1'-oxalyldiimidazole In benzene 1.) r.t., 15 min, 2.) 68-70 deg C, 1 h; | 88% |
| With acetic anhydride | |
| With acetic anhydride for 0.0833333h; Heating; |
-
-
951-82-6
3,4,5-trimethoxyphenyl acetic acid

-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

| Conditions | Yield |
|---|---|
| With sodium azide; TEA; triethylphosphine; (bis-(2-methoxyethyl)amino)sulfur trufluoride In dichloromethane; dimethyl sulfoxide at 0℃; for 50h; | 88% |
-
-
773837-37-9
sodium cyanide

-
-
3840-30-0
5-(chloromethyl)-1,2,3-trimethoxybenzene

-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 20℃; for 36h; | 85% |
| In acetonitrile Reflux; | |
| In acetonitrile for 4h; Reflux; | |
| In dimethyl sulfoxide |
-
-
10385-36-1
2,3,4-trimethoxybromobenzene

-
-
75-05-8
acetonitrile

-
A
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

-
B
-
36764-81-5
Bis-(3,4,5-trimethoxy-phenyl)-acetonitrile

| Conditions | Yield |
|---|---|
| With ethoxyethoxyethanol; sodium amide In 1,2-dimethoxyethane 1.) 45 deg C, 2 h, 2.) -10 deg C, 2 h, 3.) -30 deg C, 4.5 h; | A 83% B 11% |

-
-
21852-50-6
3,4,5-trimethoxybenzyl bromide

-
-
143-33-9
sodium cyanide

-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide Ambient temperature; | 75% |
-
-
5955-75-9
3,4,5-trimethoxybenzoyl cyanide

-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

| Conditions | Yield |
|---|---|
| With sodium sulfate; ethanethiol; zinc(II) chloride weiteres Reagens: Benzol; Erwaermen des Reaktionsprodukts mit Raney-Nickel in wss. Methanol; |

-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

| Conditions | Yield |
|---|---|
| With acetic anhydride |
-
-
139109-56-1
Oxime of 3,4,5-trimethoxyphenylpyruvic acid

-
-
108-24-7
acetic anhydride

-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile
-
-
151-50-8
potassium cyanide

-
-
86-81-7
3,4,5-trimethoxy-benzaldehyde

-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

| Conditions | Yield |
|---|---|
| With water; chloroformic acid ethyl ester; magnesium sulfate Hydrieren des Reaktionsprodukts an Palladium in Benzol; |
-
-
10385-36-1
2,3,4-trimethoxybromobenzene

-
-
75-05-8
acetonitrile

-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

| Conditions | Yield |
|---|---|
| With sodium amide 1.) liquid ammonia, 5 min, 2.) liquid ammonia, 15 min; Yield given. Multistep reaction; |
-
-
151-50-8
potassium cyanide

-
-
3840-30-0
5-(chloromethyl)-1,2,3-trimethoxybenzene

-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

| Conditions | Yield |
|---|---|
| In 1,4-dioxane; water Heating; |
-
-
42973-55-7
4-hydroxy-3,5-dimethoxyphenylacetonitrile

-
-
77-78-1
dimethyl sulfate

-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone |
-
-
143-33-9
sodium cyanide

-
-
3840-30-0
5-(chloromethyl)-1,2,3-trimethoxybenzene

-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

| Conditions | Yield |
|---|---|
| With sodium iodide |
-
-
151-50-8
potassium cyanide

-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

| Conditions | Yield |
|---|---|
| With methanol | |
| With methanol | |
| With ethanol |
-
-
13435-20-6
tetraethylammoniumcyanide

-
-
3840-30-0
5-(chloromethyl)-1,2,3-trimethoxybenzene

-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; |
-
-
3840-31-1
(3,4,5-trimethoxyphenyl)methanol

-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: SOCl2 / dioxane / 20 °C 2: acetonitrile / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 80 percent / PBr3, triethylamine / CCl4 / 1) 1h, 0 degC 2) 3h, roomtemp. 2: 75 percent / dimethylsulfoxide / Ambient temperature View Scheme | |
| Multi-step reaction with 5 steps 1: pyridinium chlorochromate / CH2Cl2 / 3 h 2: sodium acetate / acetic anhydride / Heating 3: NaOH / H2O / Heating 4: hydroxylamine hydrochloride / aq. NaOH / 0.5 h / 60 °C 5: acetic anhydride / 0.08 h / Heating View Scheme |
-
-
86-81-7
3,4,5-trimethoxy-benzaldehyde

-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: NaBH4 / methanol / 20 °C 2: SOCl2 / dioxane / 20 °C 3: acetonitrile / 20 °C View Scheme | |
| Multi-step reaction with 4 steps 1: sodium acetate / acetic anhydride / Heating 2: NaOH / H2O / Heating 3: hydroxylamine hydrochloride / aq. NaOH / 0.5 h / 60 °C 4: acetic anhydride / 0.08 h / Heating View Scheme | |
| Multi-step reaction with 3 steps 1: sodium tetrahydroborate; methanol / tetrahydrofuran / 2 h / 0 °C 2: thionyl chloride / dichloromethane / 1.5 h / 20 °C 3: dimethyl sulfoxide / 36 h / 20 °C View Scheme |
-
-
1916-07-0
3,4,5-trimethoxybenzoic acid methyl ester

-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 70 percent / LAH / tetrahydrofuran 2: 80 percent / PBr3, triethylamine / CCl4 / 1) 1h, 0 degC 2) 3h, roomtemp. 3: 75 percent / dimethylsulfoxide / Ambient temperature View Scheme | |
| Multi-step reaction with 6 steps 1: lithium aluminium hydride / diethyl ether / 3 h / Heating 2: pyridinium chlorochromate / CH2Cl2 / 3 h 3: sodium acetate / acetic anhydride / Heating 4: NaOH / H2O / Heating 5: hydroxylamine hydrochloride / aq. NaOH / 0.5 h / 60 °C 6: acetic anhydride / 0.08 h / Heating View Scheme | |
| Multi-step reaction with 3 steps 1: LiAlH4 / diethyl ether 2: HCl / benzene / 0 °C 3: dioxane; H2O / Heating View Scheme |
-
-
61404-52-2
3-(3,4,5-trimethoxyphenyl)pyruvic acid

-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: hydroxylamine hydrochloride / aq. NaOH / 0.5 h / 60 °C 2: acetic anhydride / 0.08 h / Heating View Scheme |
-
-
62616-09-5
2-Phenyl-4-(3',4',5'-trimethoxybenzylidene)oxazolone

-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: NaOH / H2O / Heating 2: hydroxylamine hydrochloride / aq. NaOH / 0.5 h / 60 °C 3: acetic anhydride / 0.08 h / Heating View Scheme |
-
-
4521-61-3
3,4,5-Trimethoxybenzoyl chloride

-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 2: ethanethiol; ZnCl2; Na2SO4 / weiteres Reagens: Benzol; Erwaermen des Reaktionsprodukts mit Raney-Nickel in wss. Methanol View Scheme |
-
-
487-11-6
elemicin

-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: ozone; ethyl acetate; oxygen / -15 °C / und anschliessende Hydrierung an Palladium/Calciumcarbonat unter Kuehlung; Isolierung als Natriumhydrogensulfit-Addukt 3: acetic acid anhydride View Scheme |
-
-
5320-31-0
3,4,5-trimethoxyphenylacetaldehyde

-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 2: acetic acid anhydride View Scheme |
-
-
91-10-1
1,3-dimethoxy-2-hydroxy-benzene

-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: H2O 2: dimethylformamide 3: K2CO3 / acetone View Scheme |
-
-
39667-14-6
2,6-dimethoxy-4-<(N,N-dimethylamino)methyl>phenol

-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: dimethylformamide 2: K2CO3 / acetone View Scheme |
-
-
3840-30-0
5-(chloromethyl)-1,2,3-trimethoxybenzene

-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

| Conditions | Yield |
|---|---|
| In water; ethyl acetate; N,N-dimethyl-formamide |
-
-
7677-24-9
trimethylsilyl cyanide

-
-
21852-50-6
3,4,5-trimethoxybenzyl bromide

-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In acetonitrile Reflux; | |
| With tetra-n-butylammoniumfluoride trihydrate In acetonitrile for 6h; Reflux; | |
| With tetrabutyl ammonium fluoride In acetonitrile for 6h; Reflux; | |
| With tetrabutyl ammonium fluoride In acetonitrile for 6h; Reflux; |
-
-
149-91-7
3,4,5-trihydroxybenzoic acid

-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: potassium carbonate / acetone / Reflux 2: lithium aluminium tetrahydride / tetrahydrofuran / 4 h / 20 °C / Inert atmosphere; Cooling with ice 3: phosphorus tribromide / dichloromethane / 4 h / Cooling with ice; Inert atmosphere 4: tetra-n-butylammoniumfluoride trihydrate / acetonitrile / 6 h / Reflux View Scheme | |
| Multi-step reaction with 4 steps 1: potassium carbonate / acetone / Reflux 2: lithium aluminium tetrahydride / tetrahydrofuran / 0 - 20 °C 3: phosphorus tribromide / dichloromethane / 0 - 20 °C 4: tetrabutyl ammonium fluoride / acetonitrile / Reflux View Scheme | |
| Multi-step reaction with 4 steps 1: potassium carbonate / acetone / 4.5 h / Reflux 2: lithium aluminium tetrahydride / tetrahydrofuran / 4 h / 20 °C / Inert atmosphere 3: phosphorus tribromide / dichloromethane / 4 h / Inert atmosphere; Cooling with ice 4: tetrabutyl ammonium fluoride / acetonitrile / 6 h / Reflux View Scheme | |
| Multi-step reaction with 4 steps 1: potassium carbonate / acetone / 4.5 h / Reflux 2: lithium aluminium tetrahydride / tetrahydrofuran / 4 h / 20 °C / Inert atmosphere; Cooling with ice 3: phosphorus tribromide / dichloromethane / 4 h / Inert atmosphere; Cooling with ice 4: tetrabutyl ammonium fluoride / acetonitrile / 6 h / Reflux View Scheme |
-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

-
-
109-94-4
formic acid ethyl ester

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol for 2h; Heating; | 98% |

| Conditions | Yield |
|---|---|
| With [carbonylchlorohydrido{bis[2-(diphenylphosphinomethyl)ethyl]amino}ethylamino] ruthenium(II); potassium tert-butylate In toluene at 135℃; for 4h; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol at 20 - 85℃; for 3h; Inert atmosphere; | 97% |
-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

-
-
111-27-3
hexan-1-ol

-
-
108781-06-2
2-(3,4,5-Trimethoxy-phenyl)-octanenitrile

| Conditions | Yield |
|---|---|
| With [carbonylchlorohydrido{bis[2-(diphenylphosphinomethyl)ethyl]amino}ethylamino] ruthenium(II); potassium tert-butylate In toluene at 135℃; for 4h; Inert atmosphere; | 97% |

| Conditions | Yield |
|---|---|
| With [carbonylchlorohydrido{bis[2-(diphenylphosphinomethyl)ethyl]amino}ethylamino] ruthenium(II); potassium tert-butylate In toluene at 135℃; for 4h; Inert atmosphere; | 96% |
-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

-
-
4559-70-0
Diphenylphosphine oxide

-
-
159386-48-8
(3,4,5-trimethoxybenzyl)diphenylphosphine oxide

| Conditions | Yield |
|---|---|
| With tris(dibenzylideneacetone)dipalladium(0) chloroform complex; potassium tert-butylate; 2'-(di-tert-butylphosphanyl)-N,N-dimethyl-[1,1'-biphenyl]-2-amine In 1,4-dioxane at 120℃; for 16h; Reagent/catalyst; Inert atmosphere; Glovebox; Schlenk technique; | 96% |
| With potassium tert-butylate; nickel dichloride In 1,4-dioxane at 120℃; for 16h; Inert atmosphere; Schlenk technique; | 71% |
-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

-
-
5779-98-6
O-methoxymethylisovanillin

| Conditions | Yield |
|---|---|
| With sodium hydroxide; Aliquat 336 In dichloromethane for 4h; Ambient temperature; | 95% |
-
-
2314-37-6
3-iodo-4-methoxybenzaldehyde

-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

-
-
1408251-07-9
C19H18INO4

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol at 45℃; | 93% |

| Conditions | Yield |
|---|---|
| With [carbonylchlorohydrido{bis[2-(diphenylphosphinomethyl)ethyl]amino}ethylamino] ruthenium(II); potassium tert-butylate In toluene at 135℃; for 10h; Inert atmosphere; Green chemistry; | 93% |
-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

-
-
951-82-6
3,4,5-trimethoxyphenyl acetic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide Heating; | 92% |
| With sulfuric acid; acetic acid In water for 22h; Reflux; | 82% |
| With hydrogenchloride | |
| With potassium hydroxide In ethanol Reflux; | |
| With water; potassium hydroxide In ethanol for 15h; Reflux; |
-
-
7521-41-7
2-Aminonicotinaldehyde

-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

-
-
1075240-58-2
3-(2,3,4-trimethoxyphenyl)-[1,8]naphthyridin-2-ylamine

| Conditions | Yield |
|---|---|
| With aminosulfonic acid for 0.05h; microwave irradiation; | 92% |
-
-
64-17-5
ethanol

-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

-
-
93270-42-9
Ethyl 3,4,5-Trimethoxyphenylacetimidate Hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride Ambient temperature; | 91% |
| With hydrogenchloride In benzene | 82% |
| With hydrogenchloride In chloroform for 2h; Ambient temperature; | |
| With hydrogenchloride In diethyl ether at 0℃; | |
| With hydrogenchloride In diethyl ether |
-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

-
-
108-94-1
cyclohexanone

| Conditions | Yield |
|---|---|
| With sodium hydroxide; tetra(n-butyl)ammonium hydrogensulfate at 0 - 15℃; for 1h; | 90% |
-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

-
-
120-14-9
3,4-dimethoxy-benzaldehyde

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol at 85℃; for 1h; Knoevenagel Condensation; Inert atmosphere; | 90% |
-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

-
-
62038-54-4
ethyl 3,4,5,-trimethoxy-α-cyanobenzeneacetate

| Conditions | Yield |
|---|---|
| 89% | |
| 89% |
-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

-
-
77-78-1
dimethyl sulfate

-
-
19589-24-3
2-methyl-2-(3,4,5-trimethoxyphenyl)propanenitrile

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran at -30 - 20℃; | 88% |
-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

-
-
22237-13-4
(4-ethoxyphenyl)boronic acid

| Conditions | Yield |
|---|---|
| With [2,2]bipyridinyl; potassium fluoride; palladium diacetate; trifluoroacetic acid In tetrahydrofuran; water at 80℃; for 2h; Inert atmosphere; Schlenk technique; | 87% |
-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

-
-
149507-26-6
3-fluoro-4-methoxy-phenylboronic acid

| Conditions | Yield |
|---|---|
| With [2,2]bipyridinyl; potassium fluoride; palladium diacetate; trifluoroacetic acid In tetrahydrofuran; water at 80℃; for 2h; Inert atmosphere; Schlenk technique; | 85% |
-
-
766-47-2
1-ethynyl-2-methylbenzene

-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; tert-butyl alcohol In dimethyl sulfoxide at 80℃; for 12h; Inert atmosphere; stereoselective reaction; | 84% |
-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

-
-
5720-05-8
4-methylphenylboronic acid

| Conditions | Yield |
|---|---|
| With [2,2]bipyridinyl; potassium fluoride; palladium diacetate; trifluoroacetic acid In tetrahydrofuran; water at 80℃; for 2h; Inert atmosphere; Schlenk technique; | 84% |
-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

-
-
32316-92-0
naphthalene-2-boronic acid

| Conditions | Yield |
|---|---|
| With [2,2]bipyridinyl; potassium fluoride; palladium diacetate; trifluoroacetic acid In tetrahydrofuran; water at 80℃; for 2h; Inert atmosphere; Schlenk technique; | 84% |

| Conditions | Yield |
|---|---|
| With [carbonylchlorohydrido{bis[2-(diphenylphosphinomethyl)ethyl]amino}ethylamino] ruthenium(II); potassium tert-butylate In toluene at 135℃; for 10h; Inert atmosphere; Green chemistry; | 83% |
-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

-
-
105-13-5
4-Methoxybenzyl alcohol

-
-
134029-62-2
(E)-3,4,4',5-tetramethoxystilbene

| Conditions | Yield |
|---|---|
| With C15H25Cl2N3NiO3; potassium tert-butylate In octane at 120℃; for 24h; Schlenk technique; Inert atmosphere; | 83% |
-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

-
-
123-11-5
4-methoxy-benzaldehyde

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol at 20℃; Reflux; | 82% |
| With sodium hydroxide; Aliquat 336 In dichloromethane for 4h; Ambient temperature; | 75% |
| Stage #1: 2-(3,4,5-trimethoxyphenyl)acetonitrile; 4-methoxy-benzaldehyde In methanol at 60℃; for 0.5h; Stage #2: With sodium methylate In methanol at 60℃; |
-
-
67-56-1
methanol

-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

-
-
91641-41-7
α-methyl-3,4,5-trimethoxybenzeneacetonitrile

| Conditions | Yield |
|---|---|
| With [carbonylchlorohydrido{bis[2-(diphenylphosphinomethyl)ethyl]amino}ethylamino] ruthenium(II); potassium tert-butylate In toluene at 135℃; for 4h; Inert atmosphere; | 82% |
-
-
13338-63-1
2-(3,4,5-trimethoxyphenyl)acetonitrile

-
-
5720-07-0
4-methoxyphenylboronic acid

-
-
203058-56-4
1-(4-methoxyphenyl)-2-(3,4,5-trimethoxyphenyl)ethane-1-one

| Conditions | Yield |
|---|---|
| With [2,2]bipyridinyl; potassium fluoride; palladium diacetate; trifluoroacetic acid In tetrahydrofuran; water at 80℃; for 2h; Inert atmosphere; Schlenk technique; | 82% |
3,4,5-Trimethoxyphenylacetonitrile Chemical Properties
Molar mass:207.2258g/mol
Structure of 3,4,5-Trimethoxyphenylacetonitrile(13338-63-1):
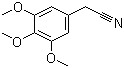
Synonyms:3,4,5-Trimethoxybenzyl cyanide;3,4,5-Trimethoxybenzylnitrile;3,4,5-Trimethoxybenzeneacetonitrile;Benzeneacetonitrile, 3,4,5-trimethoxy-;3,4,5-Trimethoxyphenylacetylnitryl
Density:1.104 g/cm3
Flash Point:132.8 °C
Boiling Point:327.9 °C at 760 mmHg
Index of Refraction:1.506
Vapour Pressure:0.000196 mmHg at 25°C
Melting point:77-79 °C(lit.)
3,4,5-Trimethoxyphenylacetonitrile Uses
3,4,5-Trimethoxyphenylacetonitrile Toxicity Data With Reference
U.S. Army Armament Research & Development Command, Chemical Systems Laboratory, NIOSH Exchange Chemicals. Vol. NX#04092.
2.Carcinogenicity:3,4,5-Trimethoxyphenylacetonitrile(13338-63-1)- Not listed as a carcinogen by NTP, IARC,ACGIH, or CA Prop 65.
Other: The toxicological properties have not been fully investigated.You can see actual entry in RTECS for complete information.
3,4,5-Trimethoxyphenylacetonitrile Safety Profile

Risk Statements:
20: Harmful by inhalation
21: Harmful in contact with skin
22: Harmful if swallowed
36: Irritating to the eyes
37: Irritating to the respiratory system
38: Irritating to the skin
Safety Statements:
24: Avoid contact with skin
25: Avoid contact with eyes
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
3,4,5-Trimethoxyphenylacetonitrile Specification
Conditions to Avoid:Incompatible materials.
Incompatibilities with Other Materials:Strong acids , strong bases,strong oxidizing agents.
Hazardous Decomposition Products: Hydrogen cyanide,carbon dioxide,nitrogen oxides, carbon monoxide.
Related Products
- 3,10-Diaminotricyclo(5.2.1.0(sup 2,6))decane
- 3,10-Dinitrophenanthrene
- 3-((10-ETHYL-11-(p-HYDROXYPHENYL)DIBENZ-(B,F)OXEPIN-3-YL)OXY)-1,2-PROPANEDIOL HYDRATE (4:1)
- 3-(1,1,2,2-Tetrafluoroethoxy)aniline
- 3-(1,1,2,2-Tetrafluoroethoxy)benzaldehyde
- 3-(1,1,2,2-Tetrafluoroethoxy)bromobenzene
- 3-(1,1,2,2-Tetrafluoroethoxy)toluene
- 3-[1,1'-Biphenyl]-4-yl-1,2,3,4-tetrahydro-1-naphthol
- 3,11-Dichloro-6,11-dihydro-6-methyldibenzo[c,f][1,2]thiazepine 5,5-dioxide
- 3-[(1,1-Dimethyl-2-hydroxyethyl)amino]-2-hydroxypropanesulfonicacid
- 1333-86-4
- 1333-88-6
- 133388-96-2
- 1333-89-7
- 13339-01-0
- 13339-02-1
- 133399-57-2
- 133407-82-6
- 133407-86-0
- 133409-72-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View