-
Name
3-CHLORODIPHENYLAMINE
- EINECS 202-922-0
- CAS No. 101-17-7
- Article Data42
- CAS DataBase
- Density 1.216 g/cm3
- Solubility
- Melting Point 112 °C(Solv: methanol (67-56-1))
- Formula C12H10ClN
- Boiling Point 337.822 °C at 760 mmHg
- Molecular Weight 203.671
- Flash Point 147.422 °C
- Transport Information
- Appearance
- Safety 28-36/37
- Risk Codes 20/21/22
-
Molecular Structure
- Hazard Symbols R20/21/22:Harmful by inhalation, in contact with skin and if swallowed.;
- Synonyms Diphenylamine,3-chloro- (6CI,8CI);N-(3-Chlorophenyl)aniline;N-(m-Chlorophenyl)aniline;Phenyl(m-chlorophenyl)amine;m-Chlorodiphenylamine;
- PSA 12.03000
- LogP 4.15660
Synthetic route

| Conditions | Yield |
|---|---|
| With tris(dibenzylideneacetone)dipalladium (0); 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; sodium t-butanolate In toluene at 90 - 110℃; for 11h; Buchwald-Hartwig reaction; | 100% |
| With pectin-stabilized copper nanoparticles In dimethyl sulfoxide at 110℃; for 2h; | 82% |

| Conditions | Yield |
|---|---|
| With dicyclohexyl-(2′,4′,6′-triisopropyl-3,6-dimethoxy-[1,1′-biphenyl]-2-yl)phosphine; chloro[2-(dicyclohexylphosphino)-3,6-dimethoxy-2',4',6'-triisopropyl-1,1'-biphenyl][2-(2-aminoethyl)phenyl]palladium(ll); sodium t-butanolate In toluene at 80℃; Inert atmosphere; | 96% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In ethanol at 75℃; for 1h; Inert atmosphere; | 95% |
| With toluene-4-sulfonic acid In ethanol at 75℃; | 40% |

| Conditions | Yield |
|---|---|
| With η5‐cyclopentadienyl‐η3‐1‐phenylallylpalladium; potassium carbonate; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In 1,4-dioxane at 80℃; for 12h; Reagent/catalyst; Temperature; Solvent; | 95% |

| Conditions | Yield |
|---|---|
| With copper immobilized at polyimide covalent organic framework In methanol; water at 20℃; for 8h; Chan-Lam Coupling; | 87% |
| With pyridine; copper(II) acetate monohydrate In dichloromethane at 39.84℃; for 24h; | 83% |
| With potassium tert-butylate; tetra(n-butyl)ammonium hydroxide; copper diacetate In water at 20℃; for 22h; | 72% |

| Conditions | Yield |
|---|---|
| Stage #1: nitrobenzene With [2,2]bipyridinyl; [MoO2Cl2(dmf)2] In toluene for 0.0333333h; Stage #2: 3-chlorophenylboronic acid With triphenylphosphine In toluene at 100℃; for 20h; | 87% |
-
-
88284-48-4
2-(trimethylsilyl)phenyl trifluoromethanesulfonate

-
-
108-42-9
3-chloro-aniline

-
-
101-17-7
3-chlorodiphenylamine

| Conditions | Yield |
|---|---|
| With cis,trans-2,5-dimethoxytetrahydrofuran; acetonedicarboxylic acid; cesium fluoride In acetonitrile at 20℃; | 85.7% |

| Conditions | Yield |
|---|---|
| With copper phthalocyanine; copper(II) sulfate In methanol at 15℃; | 85% |
| With tetrabenzoporphyrinatocobalt(II); copper diacetate In acetonitrile at 0℃; for 13h; chemoselective reaction; | 55% |

| Conditions | Yield |
|---|---|
| With copper diacetate; triethylamine at 20℃; for 24h; | 84% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-Nitrochlorobenzene With [2,2]bipyridinyl; [MoO2Cl2(dmf)2] In toluene for 0.0333333h; Stage #2: phenylboronic acid With triphenylphosphine In toluene at 100℃; for 20h; | 84% |

| Conditions | Yield |
|---|---|
| With palladium diacetate; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; sodium t-butanolate In toluene for 24h; Reflux; | 82% |
| With palladium diacetate; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; sodium t-butanolate In toluene for 24h; Reflux; | 82% |
| With palladium diacetate; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; sodium t-butanolate In toluene for 24h; Reflux; | 82% |
| With potassium hydroxide In N,N-dimethyl-formamide at 130℃; for 0.483333h; Buchwald-Hartwig Coupling; | 68% |

| Conditions | Yield |
|---|---|
| With 2,6-dimethylpyridine; tetrabutylammonium perchlorate; copper diacetate; triethylamine In acetonitrile at 20℃; for 20h; Chan-Lam Coupling; Electrochemical reaction; chemoselective reaction; | 75% |
| With N-(pyrid-2-yl)benzamide; nickel(II) acetate tetrahydrate; N,N,N',N'-tetramethylguanidine In toluene at 60℃; for 24h; Chan-Lam Coupling; | 74% |
| With ammonium cerium (IV) nitrate; copper diacetate In toluene at 20℃; for 12h; Chan-Lam Coupling; | 73% |
| With 2,6-dimethylpyridine; fac-tris(2-phenylpyridinato-N,C2')iridium(III); copper diacetate; n-tetradecanoic acid In toluene; acetonitrile at 35℃; for 20h; Reagent/catalyst; Chan-Lam Coupling; Irradiation; | 62% |
| With C30H25N4O(1-)*3C2H3O2(1-)*2Cu(2+) In methanol at 20℃; for 24h; | 34 %Spectr. |
-
-
65739-06-2
3-[(4-methylphenyl)sulfonyl]-1-phenyltriaz-1-ene

-
-
63503-60-6
3-chlorophenylboronic acid

-
-
101-17-7
3-chlorodiphenylamine

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In toluene at 110℃; for 24h; Molecular sieve; Inert atmosphere; Schlenk technique; | 65% |

| Conditions | Yield |
|---|---|
| With copper; potassium carbonate | 50% |

| Conditions | Yield |
|---|---|
| With copper; potassium carbonate; potassium iodide Reagens 4: Nitrobenzol; und Erwaermen des Reaktionsprodukts mit wss.-aethanol. HCl; | |
| With copper(l) iodide; potassium carbonate In nitrobenzene for 15h; Heating; |
-
-
13278-36-9
N-(3-chlorophenyl)anthranilic acid

-
-
101-17-7
3-chlorodiphenylamine

| Conditions | Yield |
|---|---|
| at 250 - 260℃; |
-
-
73347-61-2
N-(3-chloro-phenyl)-N-phenyl-benzamide

-
A
-
101-17-7
3-chlorodiphenylamine

-
B
-
65-85-0
benzoic acid

| Conditions | Yield |
|---|---|
| With barium dihydroxide; cetyltrimethylammonim bromide In water at 65.5℃; Rate constant; Mechanism; absence of ctab, other reagent, other solvent; |
-
-
108-90-7
chlorobenzene

-
-
622-37-7
Phenyl azide

-
A
-
101-17-7
3-chlorodiphenylamine

-
B
-
1205-71-6
N-(4-chlorophenyl)aniline

-
C
-
1205-40-9
N-phenyl-2-chloroaniline

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid at 65℃; for 2h; Yield given. Yields of byproduct given; |


-
-
101-17-7
3-chlorodiphenylamine

| Conditions | Yield |
|---|---|
| With copper(l) chloride und nachfolgenden Hydrolyse; |

| Conditions | Yield |
|---|---|
| for 0.25h; Microwave irradiation; |
-
-
349579-42-6
C15H14ClNO2

-
-
101-17-7
3-chlorodiphenylamine

| Conditions | Yield |
|---|---|
| With potassium hydroxide at 130℃; for 2h; | 138 mg |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: sodium nitrite; hydrogenchloride; sodium acetate / water / 1 h / 0 °C 1.2: 0 - 20 °C 2.1: 1,8-diazabicyclo[5.4.0]undec-7-ene / toluene / 24 h / 110 °C / Molecular sieve; Inert atmosphere; Schlenk technique View Scheme |
-
-
101-17-7
3-chlorodiphenylamine

-
-
79-04-9
chloroacetyl chloride

-
-
83254-79-9
N-chloroacetyl-N-phenyl-m-chloroaniline

| Conditions | Yield |
|---|---|
| In toluene Heating; | 95% |
| In N,N-dimethyl-formamide at 20℃; for 4h; | 89.6% |
| In benzene Heating; | |
| In toluene |
-
-
101-17-7
3-chlorodiphenylamine

-
-
88-10-8
N,N-diethylcarbamyl chloride

-
-
883726-46-3
N,N-diethyl-N'-(3-chlorophenyl)-N'-phenylurea

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide In tetrahydrofuran at 0 - 20℃; | 89% |

| Conditions | Yield |
|---|---|
| potassium hydroxide; copper In various solvent(s) at 160℃; for 27h; | 88% |
-
-
101-17-7
3-chlorodiphenylamine

-
-
917-61-3
sodium isocyanate

-
-
86576-00-3
1-(m-chlorophenyl)-1-phenylurea

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In benzene for 5h; Ambient temperature; | 84% |
3-Chlorodiphenylamine Specification
The Benzenamine,3-chloro-N-phenyl-, with the CAS registry number 101-17-7, is also known as Benzenamine, 3-chloro-N-phenyl-. Its EINECS registry number is 202-922-0. This chemical's molecular formula is C12H10ClN and molecular weight is 203.050177. Its IUPAC name is called 3-chloro-N-phenylaniline.
Physical properties of Benzenamine,3-chloro-N-phenyl-: (1)ACD/LogP: 3.86; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 3.86; (4)ACD/LogD (pH 7.4): 3.86; (5)ACD/BCF (pH 5.5): 509.23; (6)ACD/BCF (pH 7.4): 509.24; (7)ACD/KOC (pH 5.5): 3014.5; (8)ACD/KOC (pH 7.4): 3014.5; (9)#H bond acceptors: 1; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 2; (12)Index of Refraction: 1.642; (13)Molar Refractivity: 60.52 cm3; (14)Molar Volume: 167.4 cm3; (15)Surface Tension: 46.1 dyne/cm; (16)Density: 1.216 g/cm3; (17)Flash Point: 147.4 °C; (18)Enthalpy of Vaporization: 58.11 kJ/mol; (19)Boiling Point: 337.8 °C at 760 mmHg; (20)Vapour Pressure: 0.000102 mmHg at 25°C.
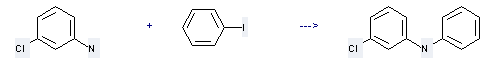
Preparation: this chemical can be prepared by 3-chloro-aniline and iodobenzene. This reaction will need reagent K2CO3, Cu. The yield is about 50%.
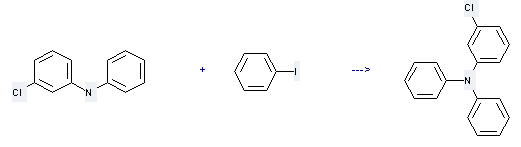
Uses of Benzenamine,3-chloro-N-phenyl-: it can be used to produce (m-chlorophenyl)diphenylamine at temperature of 160 °C. This reaction will need solvent various solvents with reaction time of 27 hours. The yield is about 88%.
When you are using this chemical, please be cautious about it as the following:
This chemical is harmful by inhalation, in contact with skin and if swallowed. After contact with your skin, you must wash immediately with plenty of ... (to be specified by the manufacturer). Whenever you will contact it, please wear suitable protective clothing and gloves.
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: C1=CC=C(C=C1)NC2=CC(=CC=C2)Cl
(2)InChI: InChI=1S/C12H10ClN/c13-10-5-4-8-12(9-10)14-11-6-2-1-3-7-11/h1-9,14H
(3)InChIKey: OHHIBZKYXJDQEU-UHFFFAOYSA-N
Related Products
- 3-Chlorodiphenylamine
- 1011-84-3
- 101184-73-0
- 101-18-8
- 10118-90-8
- 101191-83-7
- 101200-48-0
- 101200-51-5
- 1012-01-7
- 101-20-2
- 10120-28-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View