-
Name
3-Dimethylaminophenol
- EINECS 202-727-0
- CAS No. 99-07-0
- Article Data39
- CAS DataBase
- Density 1.089 g/cm3
- Solubility slightly soluble in water
- Melting Point 82-84 °C(lit.)
- Formula C8H11NO
- Boiling Point 266.5 °C at 760 mmHg
- Molecular Weight 137.181
- Flash Point 132.7 °C
- Transport Information
- Appearance brown to purple solid
- Safety 26-36-36/37/39-22
- Risk Codes 26-36-36/37/39-22
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi, Xn
Xn
- Synonyms Phenol,m-(dimethylamino)- (6CI,7CI,8CI);(3-Hydroxyphenyl)dimethylamine;3-(Dimethylamino)phenol;3-Hydroxy-N,N-dimethylaniline;3-N,N-Dimethylaminophenol;N,N-Dimethyl-m-aminophenol;NSC 62017;m-(Dimethylamino)phenol;m-(N,N-Dimethylamino)phenol;
- PSA 23.47000
- LogP 1.45820
Synthetic route
-
-
15799-79-8
3-methoxy-N,N-dimethylaniline

-
-
99-07-0
3-Dimethylaminophenol

| Conditions | Yield |
|---|---|
| With boron trichloride; tetra-(n-butyl)ammonium iodide In dichloromethane at -78 - 0℃; for 1h; dealkylation; | 95% |

| Conditions | Yield |
|---|---|
| With hydrogen In water at 200℃; under 375.038 Torr; for 3h; Reagent/catalyst; Autoclave; | 93% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; 6,7-dihydro-5H-quinolin-8-one oxime; potassium hydroxide In water at 25℃; for 24h; Inert atmosphere; | 91% |
-
-
91240-40-3
methanesulfonic acid 3-dimethylaminophenyl ester

-
-
99-07-0
3-Dimethylaminophenol

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide In tetrahydrofuran at 0℃; for 0.0166667h; | 89% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; 6,7-dihydro-5H-quinolin-8-one oxime; potassium hydroxide In water at 85℃; for 24h; Inert atmosphere; | 89% |

-
-
99-07-0
3-Dimethylaminophenol

| Conditions | Yield |
|---|---|
| In water-d2 for 0.5h; Inert atmosphere; Photolysis; | 89% |
-
-
121-69-7
N,N-dimethyl-aniline

-
A
-
619-60-3
4-(N,N-dimethylamino)phenol

-
B
-
3743-22-4
2-hydroxy-N,N-dimethylaniline

-
C
-
99-07-0
3-Dimethylaminophenol

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; antimony pentafluoride In hydrogen fluoride at -20℃; for 0.25h; Mechanism; Product distribution; | A 26% B 14% C 44% |
| With hydrogen fluoride; dihydrogen peroxide; antimony pentafluoride at -20℃; for 0.25h; | A 26% B 14% C 44% |
| With dihydrogen peroxide; antimony pentafluoride In hydrogen fluoride at -20℃; for 0.25h; | A 26% B 14% C 44% |


| Conditions | Yield |
|---|---|
| With hydrogen In methanol at 180℃; under 12751.3 Torr; for 8h; Autoclave; | 21% |
| With sodium tetrahydroborate In methanol at 20℃; |

| Conditions | Yield |
|---|---|
| at 170℃; |

| Conditions | Yield |
|---|---|
| With potassium hydroxide |

| Conditions | Yield |
|---|---|
| bei der Natronschmelze; | |
| bei der Natronschmelze; |

| Conditions | Yield |
|---|---|
| bei der Destillation unter vermindertem Druck; |
-
-
65-49-6
4-Aminosalicylic acid

-
-
77-78-1
dimethyl sulfate

-
A
-
27559-59-7
4-dimethylamino-2-hydroxy-benzoic acid methyl ester

-
B
-
99-07-0
3-Dimethylaminophenol

| Conditions | Yield |
|---|---|
| With sodium hydroxide |


| Conditions | Yield |
|---|---|
| With sulfuric acid at 55 - 60℃; und Behandeln mit Natron bei 270-300grad; | |
| Multi-step reaction with 2 steps 1: sulfuric acid 2: bei der Natronschmelze View Scheme |
-
-
506-59-2
N,N-dimethylammonium chloride

-
-
124-40-3
dimethyl amine

-
-
108-46-3
recorcinol

-
-
99-07-0
3-Dimethylaminophenol

| Conditions | Yield |
|---|---|
| With water at 200℃; |

-
-
64057-78-9
carbonic acid bis-(3-dimethylamino-phenyl ester)

-
-
62-53-3
aniline

-
A
-
603-54-3
N,N-diphenylurea

-
B
-
99-07-0
3-Dimethylaminophenol

| Conditions | Yield |
|---|---|
| at 190℃; |


| Conditions | Yield |
|---|---|
| ueber die Diazonium-Verbindung; | |
| With sulfuric acid Diazotization.und Kochen der Diazoniumsalzloesung; |
-
-
5857-93-2
2-amino-4-hydroxy-benzenesulfonic acid

-
-
74-88-4
methyl iodide

-
-
99-07-0
3-Dimethylaminophenol

| Conditions | Yield |
|---|---|
| With potassium hydroxide; water at 100 - 110℃; und Behandeln des Reaktionsprodukts mit methylschfefelsaurem Natrium in waessr. Loesung im Autoklaven auf 170-180grad; |
-
-
114-80-7
Neostigmine bromide

-
A
-
19962-04-0
Dimethylcarbaminsaeure-3-aminophenylester

-
B
-
16088-19-0
norneostigmine

-
C
-
76418-45-6
Dimethylcarbaminsaeure-3-methylaminophenylester

-
D
-
1620-19-5
3-hydroxyphenyltrimethylammonium bromide

-
E
-
99-07-0
3-Dimethylaminophenol

| Conditions | Yield |
|---|---|
| With water In various solvent(s) at 70 - 95℃; Kinetics; Thermodynamic data; Product distribution; Activation energies in 6 buffer systems with varying pH, influence of buffer concentration, ionic force, oxygen, and pilocarpine hydrochloride on the rates of hydrolysis and demethylation; |

-
-
289699-61-2
ethyl [7-(dimethylamino)-2-oxo-2H-chromen-4-yl]acetate

-
-
99-07-0
3-Dimethylaminophenol

| Conditions | Yield |
|---|---|
| at 170℃; |

| Conditions | Yield |
|---|---|
| With water at 125℃; |


-
-
99-07-0
3-Dimethylaminophenol

| Conditions | Yield |
|---|---|
| durch Destillation unter vermindertem Druck; |
-
-
7664-93-9
sulfuric acid

-
-
52177-71-6
3-(dimethylamino)phenyl carbonochloridate

-
A
-
7647-01-0
hydrogenchloride

-
B
-
124-38-9
carbon dioxide

-
C
-
99-07-0
3-Dimethylaminophenol

-
-
7647-01-0
hydrogenchloride

-
-
22440-93-3
N,N,N',N'-tetramethyl-m-phenylenediamine

-
A
-
99-07-0
3-Dimethylaminophenol

-
B
-
108-46-3
recorcinol

| Conditions | Yield |
|---|---|
| at 180℃; |

-
-
114-80-7
Neostigmine bromide

-
A
-
19962-04-0
Dimethylcarbaminsaeure-3-aminophenylester

-
B
-
16088-19-0
norneostigmine

-
C
-
76418-45-6
Dimethylcarbaminsaeure-3-methylaminophenylester

-
D
-
1620-19-5
3-hydroxyphenyltrimethylammonium bromide

-
E
-
99-07-0
3-Dimethylaminophenol

| Conditions | Yield |
|---|---|
| Heating; degradation in alkaline solution; |

-
-
60-11-7
methyl yellow

-
A
-
123-30-8
4-amino-phenol

-
B
-
121-69-7
N,N-dimethyl-aniline

-
C
-
62-53-3
aniline

-
D
-
100-23-2
N,N-Dimethyl-4-nitroaniline

-
E
-
99-07-0
3-Dimethylaminophenol

-
F
-
108-46-3
recorcinol

-
G
-
108-95-2
phenol

| Conditions | Yield |
|---|---|
| With ferrous(II) sulfate heptahydrate; sulfuric acid; water; dihydrogen peroxide at 25℃; pH=1.8; Kinetics; |

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
99-07-0
3-Dimethylaminophenol

-
-
41602-56-6
4-dimethylamino-2-hydroxy-benzaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: N,N-dimethyl-formamide With trichlorophosphate for 0.0833333h; Stage #2: 3-Dimethylaminophenol at 20 - 70℃; for 2h; | 100% |
| With methanesulfonyl chloride at 20 - 100℃; for 1h; | 90.9% |
| Stage #1: N,N-dimethyl-formamide With trichlorophosphate at 20℃; Inert atmosphere; Stage #2: 3-Dimethylaminophenol at 20 - 90℃; Vilsmeier-Haack reaction; Inert atmosphere; Stage #3: With water | 70% |

| Conditions | Yield |
|---|---|
| With silver trifluoromethanesulfonate at 60℃; for 0.05h; neat (no solvent); | 99% |
| und man destilliert das Produkt im Vakuum; |

| Conditions | Yield |
|---|---|
| With triethylamine | 99% |
-
-
100-39-0
benzyl bromide

-
-
99-07-0
3-Dimethylaminophenol

-
-
64048-42-6
N-benzyl-3-hydroxy-N,N-dimethylanilinium bromide

| Conditions | Yield |
|---|---|
| In acetonitrile | 98% |
| In acetonitrile at 20℃; |
-
-
555-16-8
4-nitrobenzaldehdye

-
-
99-07-0
3-Dimethylaminophenol

-
-
54764-79-3
6,6’-((4-nitrophenyl)methylene)bis(3-(dimethylamino)phenol)

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid; acetic acid at 60℃; for 14h; Inert atmosphere; | 98% |
-
-
1360590-90-4
C17H13F3N2O2

-
-
99-07-0
3-Dimethylaminophenol

| Conditions | Yield |
|---|---|
| With C37H34N4O3 In chloroform at 20℃; for 8h; Inert atmosphere; enantioselective reaction; | 98% |

| Conditions | Yield |
|---|---|
| With [Ru(κ1-OAc)(κ2-OAc)(κ3-1,1,1-tris(diphenylphosphinomethyl)ethane)] In acetonitrile at 70℃; for 1h; Inert atmosphere; Schlenk technique; | 98% |
-
-
99-07-0
3-Dimethylaminophenol

-
-
481666-74-4
(2-formyl-11-methoxycarbonylmethyl-14-methyl-6,7,10,11,17,18-hexahydro-9H-8,16,19-trioxa-5,11-diaza-dibenzo[a,g]cyclopentadecen-5-yl)-acetic acid methyl ester

-
-
481666-99-3
[2-(3,6-bis-dimethylamino-9H-xanthen-9-yl)-11-methoxycarbonylmethyl-14-methyl-6,7,10,11,17,18-hexahydro-9H-8,16,19-trioxa-5,11-diaza-dibenzo[a,g]cyclopentadecen-5-yl]-acetic acid methyl ester

| Conditions | Yield |
|---|---|
| toluene-4-sulfonic acid In propionic acid at 60℃; | 97% |
| With toluene-4-sulfonic acid; propionic acid |

| Conditions | Yield |
|---|---|
| toluene-4-sulfonic acid In propionic acid at 60℃; for 16h; | 97% |

| Conditions | Yield |
|---|---|
| With acetic acid at 20℃; | A 97% B n/a |


| Conditions | Yield |
|---|---|
| With 5% Rh/C; oxygen; trifluoroacetic acid at 60℃; for 34h; | 97% |
-
-
80522-42-5
triisopropylsilyl trifluoromethanesulfonate

-
-
99-07-0
3-Dimethylaminophenol

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 22h; Inert atmosphere; | 97% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-Dimethylaminophenol With potassium hydroxide In toluene at 90℃; for 1h; Reflux; Stage #2: 4-nitrophenyl dimethylcarbamate In toluene for 3h; Product distribution / selectivity; Reflux; | 96.2% |
-
-
15781-70-1
malonic acid bis-(2,4,6-trichloro-phenyl) ester

-
-
99-07-0
3-Dimethylaminophenol

-
-
64369-54-6
7-(dimethylamino)-4-hydroxy-2H-1-benzopyran-2-one

| Conditions | Yield |
|---|---|
| In toluene at 110℃; for 4h; | 96% |

| Conditions | Yield |
|---|---|
| With 1-(4-ferrocenylbutyl)-3-{3-[dihydroxy(methoxy)silyl]propyl}benzimidazol-3-ium chloride supported on Fe3O4(at)SiO2 core-shell/BiOCl nano-composite In ethanol; water at 20℃; for 0.333333h; Sonication; | 95% |
| Stage #1: 4-nitrobenzaldehdye; malononitrile With polyethyleneimine covalently bound on the surface of Fe3O4 magnetic nanoparticle by [3-(2,3-epoxypropoxy)propyl]trimethoxysilane as cross linker In water for 0.0166667h; Sonication; Green chemistry; Stage #2: 3-Dimethylaminophenol In water for 1.5h; Reagent/catalyst; Time; Temperature; Sonication; Green chemistry; | 94% |
| With mesoporous Al-MCM-41 functionalized by layered double hydroxide nanosheets and (3-aminopropyl)triethoxysilane In ethanol at 80℃; for 6h; Schlenk technique; | 91% |

-
-
99-07-0
3-Dimethylaminophenol

-
-
497235-37-7
6-chloro-1-[(4-methoxyphenyl)methyl]-4-(trifluoromethyl)hydroquinazolin-2-one

| Conditions | Yield |
|---|---|
| With C37H34N4O3 In chloroform at 20℃; for 2h; Inert atmosphere; enantioselective reaction; | 95% |
-
-
88-10-8
N,N-diethylcarbamyl chloride

-
-
99-07-0
3-Dimethylaminophenol

-
-
63907-38-0
3-<<(diethylamino)carbonyl>oxy>-N,N-dimethylaniline

| Conditions | Yield |
|---|---|
| With pyridine; triethylamine In tetrahydrofuran Reflux; Inert atmosphere; | 94% |
| With sodium hydride 1.) diethyl ether, DMF, 15 min, 2.) RT, 1.5 h; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| With 6A-{{2-{{2-[(2-aminoethyl)amino]ethyl}amino}ethyl}amino}-6A-deoxy-β-cyclodextrin In water at 20℃; for 6h; chemoselective reaction; | 94% |
| With 2-amino-2-hydroxymethyl-1,3-propanediol In ethanol; water at 20℃; for 3.5h; Green chemistry; | 83% |
| With piperidine In ethanol at 20℃; | 31% |

-
-
104-88-1
4-chlorobenzaldehyde

-
-
109-77-3
malononitrile

-
-
99-07-0
3-Dimethylaminophenol

-
-
797028-49-0
2-amino-4-(4-chlorophenyl)-7-dimethylamino-4H-chromene-3-carbonitrile

| Conditions | Yield |
|---|---|
| With 1-(4-ferrocenylbutyl)-3-{3-[dihydroxy(methoxy)silyl]propyl}benzimidazol-3-ium chloride supported on Fe3O4(at)SiO2 core-shell/BiOCl nano-composite In ethanol; water at 20℃; for 0.333333h; Sonication; | 94% |
| With mesoporous Al-MCM-41 functionalized by layered double hydroxide nanosheets and (3-aminopropyl)triethoxysilane In ethanol at 80℃; for 6h; Schlenk technique; | 90% |
| With 2-amino-2-hydroxymethyl-1,3-propanediol In ethanol; water at 20℃; for 2.5h; Green chemistry; | 85% |
| With piperidine In ethanol at 35℃; for 12h; |


| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 0 - 20℃; | 94% |
| With potassium carbonate; potassium iodide In acetone Reflux; | 81% |
| With potassium carbonate In acetonitrile at 0 - 20℃; | 43% |

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 20℃; for 8h; Nosylation; | 93% |
-
-
2942-58-7
diethyl cyanophosphonate

-
-
99-07-0
3-Dimethylaminophenol

-
-
4619-09-4
phosphoric acid diethyl ester-(3-dimethylamino-phenyl ester)

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; for 0.5h; phosphorylation; | 93% |

| Conditions | Yield |
|---|---|
| With piperidine In ethanol at 20℃; | 93% |
| With 1-(4-ferrocenylbutyl)-3-{3-[dihydroxy(methoxy)silyl]propyl}benzimidazol-3-ium chloride supported on Fe3O4(at)SiO2 core-shell/BiOCl nano-composite In ethanol; water at 20℃; for 0.466667h; Sonication; | 85% |
| With 2-amino-2-hydroxymethyl-1,3-propanediol In ethanol; water at 20℃; for 3h; Green chemistry; | 84% |

-
-
99-07-0
3-Dimethylaminophenol

-
-
481666-87-9
2-(11-carbamoylmethyl-2-formyl-14-methyl-6,7,10,11,17,18-hexahydro-9H-8,16,19-trioxa-5,11-diaza-dibenzo[a,g]cyclopentadecen-5-yl)-acetamide

| Conditions | Yield |
|---|---|
| toluene-4-sulfonic acid In propionic acid at 60℃; | 93% |
-
-
99-61-6
3-nitro-benzaldehyde

-
-
109-77-3
malononitrile

-
-
99-07-0
3-Dimethylaminophenol

-
-
339061-90-4
2-amino-7-(dimethylamino)-4-(3-nitrophenyl)-4H-chromene-3-carbonitrile

| Conditions | Yield |
|---|---|
| Stage #1: 3-nitro-benzaldehyde; malononitrile With polyethyleneimine covalently bound on the surface of Fe3O4 magnetic nanoparticle by [3-(2,3-epoxypropoxy)propyl]trimethoxysilane as cross linker In water for 0.0166667h; Sonication; Green chemistry; Stage #2: 3-Dimethylaminophenol In water for 1.5h; Sonication; Green chemistry; | 93% |
| With 1-(4-ferrocenylbutyl)-3-{3-[dihydroxy(methoxy)silyl]propyl}benzimidazol-3-ium chloride supported on Fe3O4(at)SiO2 core-shell/BiOCl nano-composite In ethanol; water at 20℃; for 0.366667h; Sonication; | 90% |
| With mesoporous Al-MCM-41 functionalized by layered double hydroxide nanosheets and (3-aminopropyl)triethoxysilane In ethanol at 80℃; for 6h; Schlenk technique; | 89% |
| With 2-amino-2-hydroxymethyl-1,3-propanediol In ethanol; water at 20℃; for 2h; Green chemistry; | 87% |
| With piperidine In ethanol at 35℃; for 12h; |


-
-
99-07-0
3-Dimethylaminophenol

| Conditions | Yield |
|---|---|
| With C37H34N4O3 In chloroform at 20℃; for 3.5h; Inert atmosphere; enantioselective reaction; | 93% |

| Conditions | Yield |
|---|---|
| With Difluoroacetic acid; 5% palladium on Al2O3; oxygen at 20℃; for 18h; | 93% |
-
-
139152-08-2
4,5-dichlorophthalonitrile

-
-
99-07-0
3-Dimethylaminophenol

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 90℃; for 18h; Inert atmosphere; | 93% |
| With potassium carbonate In N,N-dimethyl-formamide at 90℃; for 18h; | 93% |
3-Dimethylaminophenol Chemical Properties
IUPAC Name: 3-(dimethylamino)phenol
Molecular Formula: C8H11NO
Formula Weight: 137.18g/mol
Appearance: gray powder
Density: 1.089 g/cm3
Melting Point: 82 - 84 °C
Boiling Point: 266.5 °C at 760 mmHg
Refractive index: 1.589
Flash point: 132.7 °C
Vapor Pressure: 0.00524 mmHg at 25°C
Solubility: dissolved in ethanol, ether, benzene, acetone, alkali, and inorganic acid,not almost soluble in water
EINECS: 202-727-0
liansport Information: 70kgs
Freely Rotating Bonds: 2
Molar Refractivity: 42.44 cm3
Molar Volume: 125.8 cm3
Polarizability: 16.82 10-24cm3
Surface Tension: 44 dyne/cm
Enthalpy of Vaporization: 52.48 kJ/mol
Sensitive: Light Sensitive
The chemical synonyms of 3-Dimethylaminophenol (CAS NO.99-07-0) are (3-Hydroxyphenyl)dimethylamine;3-(dimethylamino)-pheno;3-Hydroxydimethylaminobenzene;3-Hydroxy-N,N-dimethylaniline and 3-n,n-dimethylaminophenol.Its product categories is benzene derivative;Aromatic Phenols;Phenoles and thiophenoles;Amines;Aromatics and Pharmaceutical Intermediate.The molecular structure of 3-Dimethylaminophenol (CAS NO.99-07-0) is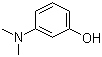 .
.
3-Dimethylaminophenol Uses
It can be used as neostigmine bromide intermediate and dye intermediate.
3-Dimethylaminophenol Production
It is derived by the Inter-amino-phenol reaction with dimethyl sulfate.
3-Dimethylaminophenol Toxicity Data With Reference
| 1. | mma-sat 33 µg/plate | EMMUEG Environmental and Molecular Mutagenesis. 11 (Suppl 12)(1988),1. |
3-Dimethylaminophenol Consensus Reports
Reported in EPA TSCA Inventory.
3-Dimethylaminophenol Safety Profile
Mutation data reported. When heated to decomposition it emits toxic vapors of NOx.
Hazard Codes:  Xi,
Xi, Xn .
Xn .
Xn: Harmful
Xi: Irritant
Risk Statements: 36/37/38-20/21/22 .
20/21/22: Harmful by inhalation, in contact with skin and if swallowed.
36/37/38: Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36-36/37/39-22.
22: Do not breathe dust.
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
36: Wear suitable protective clothing.
36/37/39: Wear suitable protective clothing, gloves and eye/face protection.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View