-
Name
4-(3-Chloro-4-fluorophenylamino)-7-methoxyquinazolin-6-ol
- EINECS 1806241-263-5
- CAS No. 184475-71-6
- Article Data51
- CAS DataBase
- Density 1.49 g/cm3
- Solubility
- Melting Point >260 °C (dec.)
- Formula C15H11ClFN3O2
- Boiling Point 478.809 °C at 760 mmHg
- Molecular Weight 319.723
- Flash Point 243.375 °C
- Transport Information
- Appearance tan solid
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 4-(3-Chloro-4-fluoroanilino)-6-hydroxy-7-methoxyquinazoline;4-(3-Chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline;N-(3-Chloro-4-fluorophenyl)-6-hydroxy-7-methoxyquinazolin-4-amine;
- PSA 67.27000
- LogP 3.95310
Synthetic route
-
-
788136-89-0
4-((3-chloro-4-fluorophenyl)amino)-7-methoxyquinazolin-6-yl acetate

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| With ammonium hydroxide In methanol at 20℃; for 13h; Inert atmosphere; | 99% |
| With sodium methylate In methanol for 3h; Reflux; | 95.3% |
| With methanol; ammonium hydroxide at 20℃; for 3h; | 95% |
-
-
367-21-5
3-chloro-4-fluorophenylamine

-
-
4637-24-5
N,N-dimethyl-formamide dimethyl acetal

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| With acetic acid In toluene at 65℃; for 7h; Solvent; Temperature; Concentration; Reagent/catalyst; Inert atmosphere; | 97% |


-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| Stage #1: 4-[(3-chloro-4-fluorophenyl)amino]-7-methoxyquinazolin-6-yl acetate hydrochloride With lithium hydroxide; water In methanol at 20℃; for 0.5h; Stage #2: With acetic acid In methanol; water | 94% |
| With lithium hydroxide; water In methanol at 20℃; for 0.5h; | 94% |
| Stage #1: 4-[(3-chloro-4-fluorophenyl)amino]-7-methoxyquinazolin-6-yl acetate hydrochloride With methanol; lithium hydroxide; water at 20℃; for 0.5h; Stage #2: With acetic acid In water | 94% |
-
-
913819-12-2
6-(benzyloxy)-N-(3-chloro-4-fluorophenyl)-7-methoxyquinazolin-4-amine

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| With methanesulfonic acid In chloroform for 5h; Reflux; | 91% |
| With trifluoroacetic acid at 75℃; for 4h; | 90% |
| With palladium 10% on activated carbon; hydrogen In methanol under 3102.97 Torr; for 24h; | 82% |
-
-
727658-04-0
N,N'-bis(3-chloro-4-fluorophenyl)formamidine

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene at 110℃; for 5h; | 84.2% |

-
-
367-21-5
3-chloro-4-fluorophenylamine

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| Stage #1: 4-chloro-7-methoxyquinazolin-6-yl acetate hydrochloride salt; 3-chloro-4-fluorophenylamine In N,N-dimethyl-formamide at 80℃; for 1h; Stage #2: With ammonia; water In methanol for 2h; Reflux; | 75% |

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In dichloromethane at 20℃; not specified; | 44% |

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: propan-2-ol / 5 h / 90 °C 2: 90 percent / ammonia / methanol; H2O / 24 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: toluene; isopropyl alcohol / 3 h / 20 - 65 °C 2: lithium hydroxide / water; methanol / 1 h / 20 °C View Scheme |
-
-
179688-53-0
6-acetoxy-7-methoxy-3,4-dihydroquinazolin-4-one

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 92 percent / N,N-diethylaniline; phosphoryl chloride / 1 h / 80 - 100 °C 2: propan-2-ol / 5 h / 90 °C 3: 90 percent / ammonia / methanol; H2O / 24 h / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1: SOCl2 2: NH4OH / methanol View Scheme | |
| Multi-step reaction with 3 steps 1: trichlorophosphate / toluene / Reflux 2: pyridine / isopropyl alcohol / Reflux 3: water; sodium hydroxide / methanol View Scheme |
-
-
13794-72-4
3H-6,7-dimethoxyquinazolin-4-one

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: L-methionine; methanesulfonic acid / 12 h / Heating 2: pyridine; 4-(dimethylamino)pyridine / 6 h / 100 °C 3: 92 percent / N,N-diethylaniline; phosphoryl chloride / 1 h / 80 - 100 °C 4: propan-2-ol / 5 h / 90 °C 5: 90 percent / ammonia / methanol; H2O / 24 h / 20 °C View Scheme | |
| Multi-step reaction with 5 steps 1: methionine; MeSO3H / 3 h / 100 °C 2: pyridine 3: SOCl2 4: NH4OH / methanol View Scheme | |
| Multi-step reaction with 5 steps 1: methanesulfonic acid; L-methionine / 120 °C 2: pyridine; dmap / 100 °C 3: trichlorophosphate / toluene / Reflux 4: pyridine / isopropyl alcohol / Reflux 5: water; sodium hydroxide / methanol View Scheme |
-
-
179688-52-9
6-hydroxy-7-methoxyquinazolin-4(3H)-one

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: pyridine; 4-(dimethylamino)pyridine / 6 h / 100 °C 2: 92 percent / N,N-diethylaniline; phosphoryl chloride / 1 h / 80 - 100 °C 3: propan-2-ol / 5 h / 90 °C 4: 90 percent / ammonia / methanol; H2O / 24 h / 20 °C View Scheme | |
| Multi-step reaction with 4 steps 1: pyridine 2: SOCl2 3: NH4OH / methanol View Scheme | |
| Multi-step reaction with 4 steps 1: pyridine; dmap / 100 °C 2: trichlorophosphate / toluene / Reflux 3: pyridine / isopropyl alcohol / Reflux 4: water; sodium hydroxide / methanol View Scheme |
-
-
230955-75-6
6-acetoxy-4-chloro-7-methoxyquinazoline

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: NH4OH / methanol View Scheme | |
| Multi-step reaction with 2 steps 1: pyridine / isopropyl alcohol / Reflux 2: water; sodium hydroxide / methanol View Scheme | |
| Multi-step reaction with 2 steps 1: isopropyl alcohol / 5 h / 88 °C 2: water; sodium hydroxide / methanol / 6 h / 20 °C View Scheme |
-
-
20323-74-4
2-carboethoxy-4,5-dimethoxyaniline

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 160 - 170 °C 2: methanesulfonic acid; L-methionine / 120 °C 3: pyridine; dmap / 100 °C 4: trichlorophosphate / toluene / Reflux 5: pyridine / isopropyl alcohol / Reflux 6: water; sodium hydroxide / methanol View Scheme | |
| Multi-step reaction with 6 steps 1: 165 - 170 °C 2: L-methionine; methanesulfonic acid 3: pyridine / 100 °C 4: thionyl chloride; N,N-dimethyl-formamide / 90 °C 5: isopropyl alcohol / Reflux 6: ammonia / water; methanol / 24 h / 20 °C View Scheme |
-
-
100905-33-7
ethyl 2-nitro-4,5-dimethoxybenzoate

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: palladium on activated charcoal; hydrogen 2: 160 - 170 °C 3: methanesulfonic acid; L-methionine / 120 °C 4: pyridine; dmap / 100 °C 5: trichlorophosphate / toluene / Reflux 6: pyridine / isopropyl alcohol / Reflux 7: water; sodium hydroxide / methanol View Scheme | |
| Multi-step reaction with 7 steps 1: iron; hydrogenchloride / ethanol; water / Reflux 2: 165 - 170 °C 3: L-methionine; methanesulfonic acid 4: pyridine / 100 °C 5: thionyl chloride; N,N-dimethyl-formamide / 90 °C 6: isopropyl alcohol / Reflux 7: ammonia / water; methanol / 24 h / 20 °C View Scheme |
-
-
3943-77-9
ethyl veratrate

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: nitric acid; acetic acid / 0 - 5 °C 2: palladium on activated charcoal; hydrogen 3: 160 - 170 °C 4: methanesulfonic acid; L-methionine / 120 °C 5: pyridine; dmap / 100 °C 6: trichlorophosphate / toluene / Reflux 7: pyridine / isopropyl alcohol / Reflux 8: water; sodium hydroxide / methanol View Scheme | |
| Multi-step reaction with 8 steps 1: nitric acid / 0 °C 2: iron; hydrogenchloride / ethanol; water / Reflux 3: 165 - 170 °C 4: L-methionine; methanesulfonic acid 5: pyridine / 100 °C 6: thionyl chloride; N,N-dimethyl-formamide / 90 °C 7: isopropyl alcohol / Reflux 8: ammonia / water; methanol / 24 h / 20 °C View Scheme |
-
-
93-07-2
Veratric acid

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1: sulfuric acid / Reflux 2: nitric acid; acetic acid / 0 - 5 °C 3: palladium on activated charcoal; hydrogen 4: 160 - 170 °C 5: methanesulfonic acid; L-methionine / 120 °C 6: pyridine; dmap / 100 °C 7: trichlorophosphate / toluene / Reflux 8: pyridine / isopropyl alcohol / Reflux 9: water; sodium hydroxide / methanol View Scheme | |
| Multi-step reaction with 9 steps 1: sulfuric acid 2: nitric acid / 0 °C 3: iron; hydrogenchloride / ethanol; water / Reflux 4: 165 - 170 °C 5: L-methionine; methanesulfonic acid 6: pyridine / 100 °C 7: thionyl chloride; N,N-dimethyl-formamide / 90 °C 8: isopropyl alcohol / Reflux 9: ammonia / water; methanol / 24 h / 20 °C View Scheme |
-
-
5653-40-7
2-Amino-4,5-dimethoxybenzoic acid

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: trimethyl orthoformate / methanol / 4 h / 70 °C / Reflux 2.1: methanesulfonic acid; DL-methionine / 3 h / 130 °C / Cooling with ice 2.2: pH 7 3.1: pyridine / 3 h / 20 - 100 °C 4.1: thionyl chloride / N,N-dimethyl-formamide / 3 h / 70 °C 5.1: isopropyl alcohol / 5 h / 88 °C 6.1: water; sodium hydroxide / methanol / 6 h / 20 °C View Scheme | |
| Multi-step reaction with 6 steps 1: trimethyl orthoformate / methanol / 4 h / 70 °C 2: DL-methionine; methanesulfonic acid / 3 h / 130 °C 3: pyridine / 3 h / 100 °C 4: thionyl chloride / N,N-dimethyl-formamide / 3 h / 70 °C 5: isopropyl alcohol / 5 h / 88 °C 6: sodium hydroxide; methanol / 6 h / 20 °C View Scheme | |
| Multi-step reaction with 6 steps 1: water / Microwave irradiation 2: magnesium sulfate; methanesulfonic acid 3: pyridine / 20 °C 4: triethylamine; trichlorophosphate / acetonitrile / 80 °C 5: 1,4-dioxane 6: sodium hydroxide / water; tetrahydrofuran / 4 h / 20 °C View Scheme |
-
-
26759-46-6
methyl 2-amino-4,5-dimethoxybenzoate

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: formamide / 2 h / 190 - 200 °C 2: methanesulfonic acid; L-methionine / 5 h / 150 - 160 °C 3: pyridine / 3 h / Heating / reflux 4: trichlorophosphate / 3 h / Heating / reflux 5: isopropyl alcohol / 3 h / Heating / reflux 6: methanol; lithium hydroxide; water / 0.5 h / 20 °C View Scheme | |
| Multi-step reaction with 6 steps 1: methanol; N,N-dimethyl-formamide / Inert atmosphere 2: methanesulfonic acid; DL-methionine / Inert atmosphere 3: pyridine / Inert atmosphere 4: thionyl chloride / N,N-dimethyl-formamide / Inert atmosphere 5: isopropyl alcohol / Inert atmosphere 6: ammonium hydroxide / 3 h / Inert atmosphere; Reflux View Scheme |
-
-
367-21-5
3-chloro-4-fluorophenylamine

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: triethylamine; trichlorophosphate / toluene / 20 - 60 °C 1.2: 2 h / 65 °C 2.1: lithium hydroxide; water / methanol / 0.5 h / 20 °C View Scheme |
-
-
286371-65-1
6-(benzyloxy)-4-chloro-7-methoxyquinazoline

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: N-ethyl-N,N-diisopropylamine / isopropyl alcohol / 2 h / 60 - 65 °C 2: methanesulfonic acid / chloroform / 5 h / Reflux View Scheme | |
| Multi-step reaction with 2 steps 1: isopropyl alcohol / Reflux 2: trifluoroacetic acid / 1 h / Reflux View Scheme |
-
-
621-59-0
isovanillin

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1.1: aminosulfonic acid; sodium chlorite / water / 2 h / 5 - 8 °C 2.1: hydrogenchloride / 4 h / 50 - 55 °C 3.1: potassium carbonate; potassium iodide / acetone / 25 - 30 °C 3.2: Reflux 4.1: nitric acid; acetic acid / 14 h / 25 - 35 °C 5.1: acetic acid; iron / 50 - 60 °C 6.1: ethyl acetate / 12 h / Reflux 7.1: oxalyl dichloride; N-ethyl-N,N-diisopropylamine / chloroform / 12 h / 60 - 65 °C 8.1: N-ethyl-N,N-diisopropylamine / isopropyl alcohol / 2 h / 60 - 65 °C 9.1: methanesulfonic acid / chloroform / 5 h / Reflux View Scheme | |
| Multi-step reaction with 7 steps 1: sodium formate; formic acid; hydroxyammonium sulfate / 85 °C 2: potassium carbonate / acetonitrile / 1.5 h / Reflux 3: acetic acid; nitric acid / 1 h / Cooling with ice 4: tin(ll) chloride; hydrogenchloride / water; acetic acid / 1 h / 60 °C 5: acetic acid / benzene / 2 h / 105 °C / Dean-Stark 6: acetic acid / 3 h / 130 - 140 °C 7: palladium 10% on activated carbon; hydrogen / methanol / 24 h / 3102.97 Torr View Scheme |
-
-
645-08-9
Isovanillic acid

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: hydrogenchloride / 4 h / 50 - 55 °C 2.1: potassium carbonate; potassium iodide / acetone / 25 - 30 °C 2.2: Reflux 3.1: nitric acid; acetic acid / 14 h / 25 - 35 °C 4.1: acetic acid; iron / 50 - 60 °C 5.1: ethyl acetate / 12 h / Reflux 6.1: oxalyl dichloride; N-ethyl-N,N-diisopropylamine / chloroform / 12 h / 60 - 65 °C 7.1: N-ethyl-N,N-diisopropylamine / isopropyl alcohol / 2 h / 60 - 65 °C 8.1: methanesulfonic acid / chloroform / 5 h / Reflux View Scheme | |
| Multi-step reaction with 8 steps 1: sulfuric acid / methanol / Reflux 2: potassium carbonate / butanone / Reflux 3: nitric acid; acetic acid / 30 °C 4: iron; ammonium chloride / methanol; water / Reflux 5: acetic acid; formamide / Reflux 6: trichlorophosphate; N,N-dimethyl-formamide / Reflux 7: isopropyl alcohol / Reflux 8: trifluoroacetic acid / 1 h / Reflux View Scheme |
-
-
6702-50-7
3-hydroxy-4-methoxybenzoate

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: potassium carbonate; potassium iodide / acetone / 25 - 30 °C 1.2: Reflux 2.1: nitric acid; acetic acid / 14 h / 25 - 35 °C 3.1: acetic acid; iron / 50 - 60 °C 4.1: ethyl acetate / 12 h / Reflux 5.1: oxalyl dichloride; N-ethyl-N,N-diisopropylamine / chloroform / 12 h / 60 - 65 °C 6.1: N-ethyl-N,N-diisopropylamine / isopropyl alcohol / 2 h / 60 - 65 °C 7.1: methanesulfonic acid / chloroform / 5 h / Reflux View Scheme | |
| Multi-step reaction with 7 steps 1: potassium carbonate / butanone / Reflux 2: nitric acid; acetic acid / 30 °C 3: iron; ammonium chloride / methanol; water / Reflux 4: acetic acid; formamide / Reflux 5: trichlorophosphate; N,N-dimethyl-formamide / Reflux 6: isopropyl alcohol / Reflux 7: trifluoroacetic acid / 1 h / Reflux View Scheme |
-
-
57535-57-6
methyl 3-(benzyloxy)-4-methoxy-benzoate

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: nitric acid; acetic acid / 14 h / 25 - 35 °C 2: acetic acid; iron / 50 - 60 °C 3: ethyl acetate / 12 h / Reflux 4: oxalyl dichloride; N-ethyl-N,N-diisopropylamine / chloroform / 12 h / 60 - 65 °C 5: N-ethyl-N,N-diisopropylamine / isopropyl alcohol / 2 h / 60 - 65 °C 6: methanesulfonic acid / chloroform / 5 h / Reflux View Scheme | |
| Multi-step reaction with 6 steps 1: nitric acid; acetic acid / 30 °C 2: iron; ammonium chloride / methanol; water / Reflux 3: acetic acid; formamide / Reflux 4: trichlorophosphate; N,N-dimethyl-formamide / Reflux 5: isopropyl alcohol / Reflux 6: trifluoroacetic acid / 1 h / Reflux View Scheme |
-
-
164161-49-3
methyl 5-(benzyloxy)-4-methoxy-2-nitrobenzoate

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: acetic acid; iron / 50 - 60 °C 2: ethyl acetate / 12 h / Reflux 3: oxalyl dichloride; N-ethyl-N,N-diisopropylamine / chloroform / 12 h / 60 - 65 °C 4: N-ethyl-N,N-diisopropylamine / isopropyl alcohol / 2 h / 60 - 65 °C 5: methanesulfonic acid / chloroform / 5 h / Reflux View Scheme | |
| Multi-step reaction with 5 steps 1: iron; ammonium chloride / methanol; water / Reflux 2: acetic acid; formamide / Reflux 3: trichlorophosphate; N,N-dimethyl-formamide / Reflux 4: isopropyl alcohol / Reflux 5: trifluoroacetic acid / 1 h / Reflux View Scheme |
-
-
855793-63-4
methyl 2-amino-5-(benzyloxy)-4-methoxybenzoate

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: ethyl acetate / 12 h / Reflux 2: oxalyl dichloride; N-ethyl-N,N-diisopropylamine / chloroform / 12 h / 60 - 65 °C 3: N-ethyl-N,N-diisopropylamine / isopropyl alcohol / 2 h / 60 - 65 °C 4: methanesulfonic acid / chloroform / 5 h / Reflux View Scheme | |
| Multi-step reaction with 4 steps 1: acetic acid; formamide / Reflux 2: trichlorophosphate; N,N-dimethyl-formamide / Reflux 3: isopropyl alcohol / Reflux 4: trifluoroacetic acid / 1 h / Reflux View Scheme |
-
-
286371-64-0
6-(benzyloxy)-7-methoxyquinazolin-4(3H)-one

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: oxalyl dichloride; N-ethyl-N,N-diisopropylamine / chloroform / 12 h / 60 - 65 °C 2: N-ethyl-N,N-diisopropylamine / isopropyl alcohol / 2 h / 60 - 65 °C 3: methanesulfonic acid / chloroform / 5 h / Reflux View Scheme | |
| Multi-step reaction with 3 steps 1: trichlorophosphate; N,N-dimethyl-formamide / Reflux 2: isopropyl alcohol / Reflux 3: trifluoroacetic acid / 1 h / Reflux View Scheme |

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: acetic acid / 3 h / 130 - 140 °C 2: palladium 10% on activated carbon; hydrogen / methanol / 24 h / 3102.97 Torr View Scheme |
-
-
52805-46-6
2-methoxy-5-cyanophenol

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: potassium carbonate / acetonitrile / 1.5 h / Reflux 2: acetic acid; nitric acid / 1 h / Cooling with ice 3: tin(ll) chloride; hydrogenchloride / water; acetic acid / 1 h / 60 °C 4: acetic acid / benzene / 2 h / 105 °C / Dean-Stark 5: acetic acid / 3 h / 130 - 140 °C 6: palladium 10% on activated carbon; hydrogen / methanol / 24 h / 3102.97 Torr View Scheme | |
| Multi-step reaction with 4 steps 1: potassium carbonate / methanol / 1 h / 65 °C / Inert atmosphere 2: nitric acid; acetic acid / 0 - 25 °C / Inert atmosphere 3: palladium 10% on activated carbon; hydrogen / methanol / 1.5 h / 20 °C / 1520.1 - 2280.15 Torr 4: acetic acid / toluene / 7 h / 65 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 5 steps 1: potassium carbonate / methanol / 1 h / 65 °C / Inert atmosphere 2: nitric acid; acetic acid / 0 - 25 °C / Inert atmosphere 3: tetrabutylammomium bromide; sodium dithionite; water / methanol / 30 °C / Inert atmosphere 4: acetic acid / 110 °C / Inert atmosphere 5: trifluoroacetic acid / 4 h / 75 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: potassium carbonate / acetonitrile / 0.17 h / 20 °C 1.2: 5 h / Reflux 2.1: acetic acid; nitric acid / 1 h / Cooling with ice 3.1: palladium 10% on activated carbon; hydrogen / methanol / 20 °C / 2327.23 Torr 4.1: toluene-4-sulfonic acid / toluene / 5 h / 110 °C View Scheme |
-
-
106-96-7
propargyl bromide

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

-
-
1214731-77-7
4-(3'-chloro-4'-fluoroanilino)-6-(prop-2-ynyloxy)-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 12h; Inert atmosphere; | 99% |
-
-
217817-01-1
tert-butyl 3-[2-[2-[2-(4-methylphenyl)sulfonyloxyethoxy]ethoxy]ethoxy]propanoate

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 1h; | 98.5% |
-
-
1012057-56-5
(E)-methyl 3-(4-(2-(tosyloxy)ethoxy)phenyl)acrylate

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

-
-
1012057-57-6
(E)-methyl 3-(4-(2-(4-(3-chloro-4-fluorophenylamino)-7-methoxyquinazolin-6-yloxy)ethoxy)phenyl) acrylate

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 24h; | 98% |
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 24h; | 98% |
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 24h; | 98% |
-
-
76-05-1
trifluoroacetic acid

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

-
-
655225-02-8
tert-butyl 4-(3-bromopropyl)-piperazine-1-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: 4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline; tert-butyl 4-(3-bromopropyl)-piperazine-1-carboxylate With potassium carbonate In N,N-dimethyl-formamide at 80℃; Stage #2: trifluoroacetic acid In methanol; water | 97% |

-
-
358-23-6
trifluoromethylsulfonic anhydride

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

-
-
451494-86-3
4-(3'-chloro-4'-fluorophenylamino)-7-methoxy-6-(trifluoromethanesulfonyloxy)quinazoline

| Conditions | Yield |
|---|---|
| With pyridine at 0 - 20℃; Inert atmosphere; | 94% |
| With pyridine at -6 - 20℃; | 62% |
-
-
6780-38-7
N-phthaloylglycine chloride

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| With dmap In dichloromethane at 20℃; for 4.5h; Inert atmosphere; Cooling with ice; | 93.2% |
-
-
4286-55-9
1-bromo-6-hexanol

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

-
-
1012057-92-9
6-(4-(3-chloro-4-fluorophenylamino)-7-methoxyquinazolin-6-yloxy)hexan-1-ol

| Conditions | Yield |
|---|---|
| Stage #1: 4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline With potassium carbonate In N,N-dimethyl-formamide at 40℃; for 0.166667h; Stage #2: 1-bromo-6-hexanol In N,N-dimethyl-formamide at 60℃; for 6h; | 93% |
-
-
939056-49-2
methanesulfonic acid 2-[2-(2-{2-[3-(2,2,2-trifluoro-1,1-bis-trifluoromethyl-ethoxy)-2,2-bis-(2,2,2-trifluoro-1,1-bis-trifluoromethyl-ethoxymethyl)-propoxy]-ethoxy}-ethoxy)-ethoxy]-ethyl ester

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 12h; | 93% |

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 130℃; for 6h; | 92.8% |
| With potassium carbonate In N,N-dimethyl-formamide at 130℃; for 6h; | 66% |
-
-
7357-67-7
4-(3-chloropropyl)morpholine

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

-
-
184475-35-2
gefitinib

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether; potassium carbonate; sodium iodide In N,N-dimethyl-formamide at 60℃; for 3h; Reagent/catalyst; Temperature; | 92% |
| With tetrabutylammomium bromide; sodium carbonate; potassium iodide In iso-butanol Reflux; | 90% |
| In N,N-dimethyl-formamide at 80 - 90℃; Concentration; | 88% |
-
-
627-30-5
1-chloro-3-hydroxypropane

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

-
-
1221965-74-7
M 527301

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetonitrile for 12h; Reflux; | 91% |

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 130℃; for 6h; | 89.8% |
| With potassium carbonate In N,N-dimethyl-formamide at 130℃; for 6h; | 66% |
-
-
957621-67-9
3-morpholinopropyl 4-methylbenzenesulfonate

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

-
-
184475-35-2
gefitinib

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 80℃; for 6h; Solvent; Temperature; | 89.5% |
-
-
1018895-28-7
methanesulfonic acid 3-morpholin-4-yl-propyl ester

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

-
-
184475-35-2
gefitinib

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 80℃; for 6h; Solvent; Temperature; | 89.2% |
-
-
109-70-6
1,3-chlorobromopropane

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

-
-
912556-91-3
6-(3-chloropropoxy)-N-(3-chloro-4-fluorophenyl)-7-methoxyquinazolin-4-ylamine

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In N,N-dimethyl-formamide at 40℃; for 6h; | 89% |
| With potassium carbonate; potassium iodide In N,N-dimethyl-formamide at 40℃; for 6h; | 89% |
| With potassium carbonate In N,N-dimethyl-formamide at 90℃; for 4h; | |
| With potassium carbonate In N,N-dimethyl-formamide at 90℃; for 4h; Heating; | Ca. 330 mg |
-
-
14660-52-7
ethyl 5-bromovalerate

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 40℃; for 0.5h; | 88% |
-
-
7357-67-7
4-(3-chloropropyl)morpholine

-
-
107-21-1
ethylene glycol

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 70 - 80℃; for 8h; | 85.9% |

-
-
106-95-6
allyl bromide

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 90℃; for 4h; | 82% |
-
-
2969-81-5
4-bromoethylbutanoate

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

-
-
1012057-14-5
ethyl 4-(4-(3-chloro-4-fluorophenylamino)-7-methoxyquinazolin-6-yloxy)butanoate

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 40℃; for 0.5h; | 81% |

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; Inert atmosphere; | 80% |

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

-
-
1438075-59-2
6-(3-(4-benzylhexahydropyrrolo[3,4-b][1,4]oxazin-6(2H)-yl)propoxy)-N-(3-chloro-4-fluorophenyl)-7-methoxyquinazolin-4-amine

| Conditions | Yield |
|---|---|
| With tetra-(n-butyl)ammonium iodide; potassium carbonate In N,N-dimethyl-formamide at 90℃; for 18h; | 79.59% |
| With tetra-(n-butyl)ammonium iodide; potassium carbonate In N,N-dimethyl-formamide at 90℃; for 18h; | 79.59% |
-
-
86651-36-7
2-bromoethyl 2,3,4-tri-O-acetyl-β-D-xylopyranoside

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

| Conditions | Yield |
|---|---|
| Stage #1: 4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 0.333333h; Stage #2: 2-bromoethyl 2,3,4-tri-O-acetyl-β-D-xylopyranoside In N,N-dimethyl-formamide at 80℃; for 8h; Inert atmosphere; | 79% |
-
-
125422-83-5
4 (3-bromopropyl)-morpholine

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

-
-
184475-35-2
gefitinib

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 70℃; for 12h; | 78% |
-
-
1438081-50-5
2-(3-chloropropyl)octahydrocyclopenta[c]pyrrole

-
-
184475-71-6
4-(3-chloro-4-fluorophenylamino)-6-hydroxy-7-methoxyquinazoline

-
-
1438073-01-8
N-(3-chloro-4-fluorophenyl)-6-(3-(hexahydrocyclopenta[c]pyrrol-2(1H)-yl)propoxy)-7-methoxyquinazolin-4-amine

| Conditions | Yield |
|---|---|
| With tetra-(n-butyl)ammonium iodide; potassium carbonate In N,N-dimethyl-formamide at 90℃; for 12h; | 75.59% |
| With tetra-(n-butyl)ammonium iodide; potassium carbonate In N,N-dimethyl-formamide at 90℃; for 12h; Inert atmosphere; |
4-(3-Chloro-4-fluorophenylamino)-7-methoxyquinazolin-6-ol Chemical Properties
Following is the structure of 4-(3-Chloro-4-fluorophenylamino)-7-methoxyquinazolin-6-ol (CAS NO.184475-71-6):
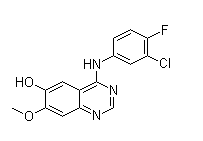
Empirical Formula: C15H11ClFN3O2
Molecular Weight: 319.7181
Index of Refraction: 1.702
Molar Refractivity: 83.101 cm3
Molar Volume: 214.63 cm3
Density: 1.49 g/cm3
Flash Point: 243.375 °C
Melting point: >260 °C (dec.)
Polarizability: 32.944 10-24cm3
Surface Tension: 64.15 dyne/cm
Enthalpy of Vaporization: 77.132 kJ/mol
Boiling Point: 478.809 °C at 760 mmHg
Appearance of 4-(3-Chloro-4-fluorophenylamino)-7-methoxyquinazolin-6-ol (CAS NO.184475-71-6): Tan Solid
Product Categories: Various Metabolites and Impurities; Intermediates & Fine Chemicals; Metabolites & Impurities; Pharmaceuticals
SMILES: Fc1ccc(cc1Cl)Nc3ncnc2c3cc(O)c(OC)c2
InChI: InChI=1/C15H11ClFN3O2/c1-22-14-6-12-9(5-13(14)21)15(19-7-18-12)20-8-2-3-11(17)10(16)4-8/h2-7,21H,1H3,(H,18,19,20)
InChIKey: JLVTVCRXFMLUIF-UHFFFAOYAN
4-(3-Chloro-4-fluorophenylamino)-7-methoxyquinazolin-6-ol Uses
4-(3-Chloro-4-fluorophenylamino)-7-methoxyquinazolin-6-ol (CAS NO.184475-71-6) can be uesd as a metabolite of Gefitinib.
4-(3-Chloro-4-fluorophenylamino)-7-methoxyquinazolin-6-ol Specification
4-(3-Chloro-4-fluorophenylamino)-7-methoxyquinazolin-6-ol , its cas register number 184475-71-6. It also can be called N-(3-Chloro-4-fluorophenyl)-6-hydroxy-7-methoxyquinazolin-4-amine ; and 6-Quinazolinol, 4-[(3-chloro-4-fluorophenyl)amino]-7-methoxy- .
Related Products
- 4-(3-Chloro-4-fluorophenylamino)-7-methoxyquinazolin-6-ol
- 18448-47-0
- 18449-41-7
- 18450-27-6
- 1845-25-6
- 18453-07-1
- 184537-03-9
- 18454-12-1
- 1845-44-9
- 18455-25-9
- 18456-87-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View